What is the molecular formula (C₂H₆ or C₂H₄) of a compound if the empirical formula is CH₂?
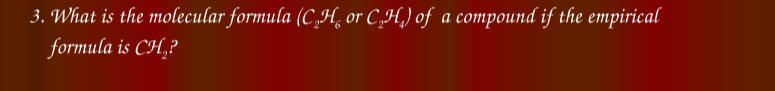
Understand the Problem
The question asks to identify the molecular formula of a compound given that its empirical formula is CH₂. The choices for the molecular formula may be C₂H₆ or C₂H₄.
Answer
The molecular formula is C₂H₄.
If the empirical formula is CH₂, the molecular formula is C₂H₄.
Answer for screen readers
If the empirical formula is CH₂, the molecular formula is C₂H₄.
More Information
The molecular formula represents the actual number of atoms in a molecule, while the empirical formula represents the simplest whole number ratio of atoms in a compound.
Tips
A common mistake is to confuse empirical and molecular formulas. The empirical formula is the simplest ratio, whereas the molecular formula is the actual number of atoms.
Sources
AI-generated content may contain errors. Please verify critical information