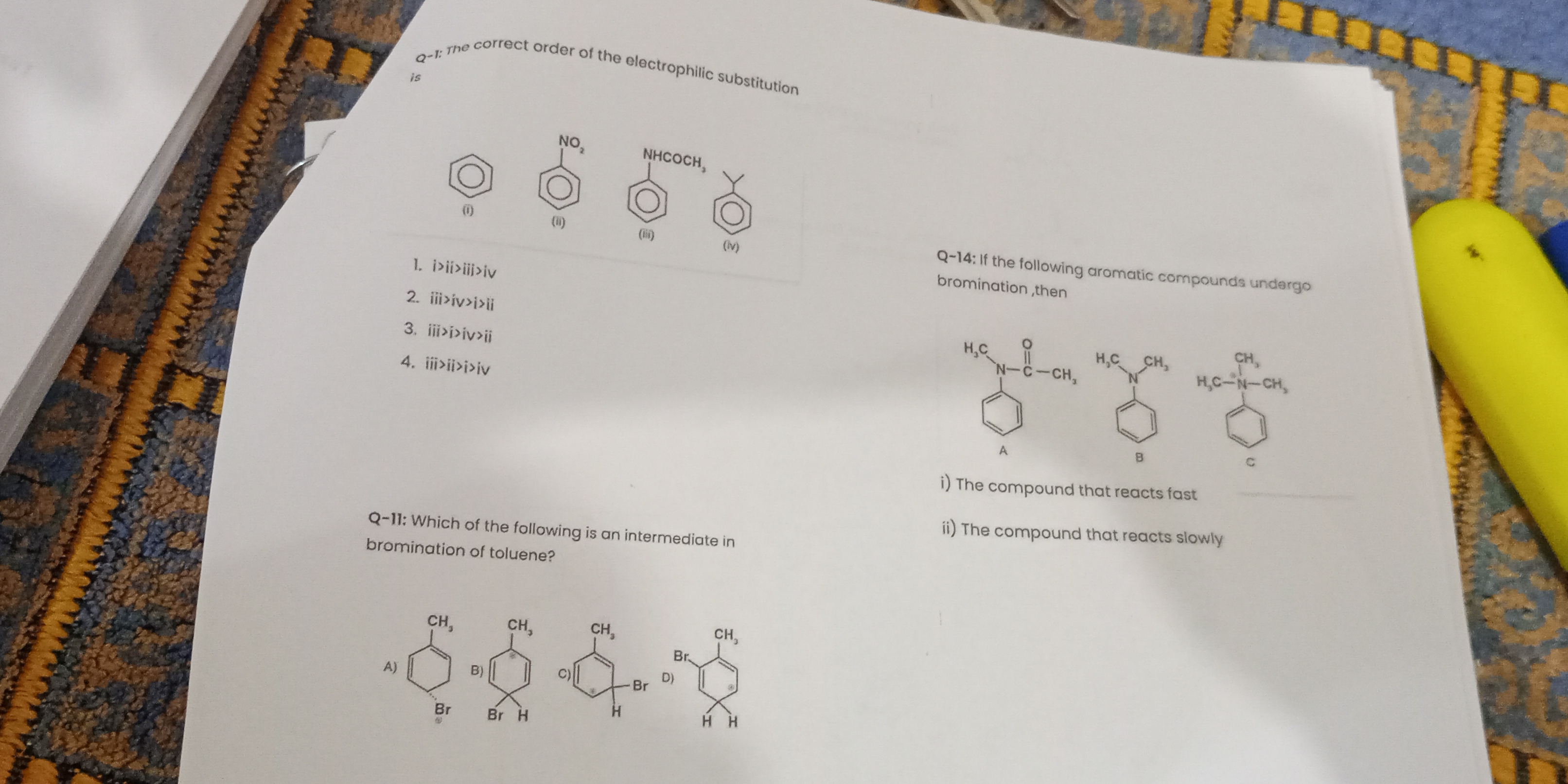
What is the correct order of the electrophilic substitution and which aromatic compounds react fast or slowly during bromination?
Understand the Problem
The question is asking about the correct order of electrophilic substitution reactions for given aromatic compounds and which compounds react fast or slowly during bromination, specifically in the context of aromatic chemistry.
Answer
iii > i > iv > ii for substitution order; Compound C reacts fast, A reacts slowly in bromination.
For electrophilic substitution: iii > i > iv > ii. For bromination rate: Compound C reacts fast, Compound A reacts slowly.
Answer for screen readers
For electrophilic substitution: iii > i > iv > ii. For bromination rate: Compound C reacts fast, Compound A reacts slowly.
More Information
In electrophilic aromatic substitution, activating groups (like NHCOCH3) make reactions faster, while deactivating groups (like NO2) slow them down. Bromination rates depend on the strength of the activating or deactivating groups present.
Tips
A common mistake is confusing the effects of activating and deactivating groups; remember that activating groups speed up reactions while deactivating groups slow them down.
Sources
- Activating and Deactivating Groups - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information