What are the properties of elements 1 to 30, including atomic number, symbol, atomic mass, electronic configuration, valence electrons, and valency?
Understand the Problem
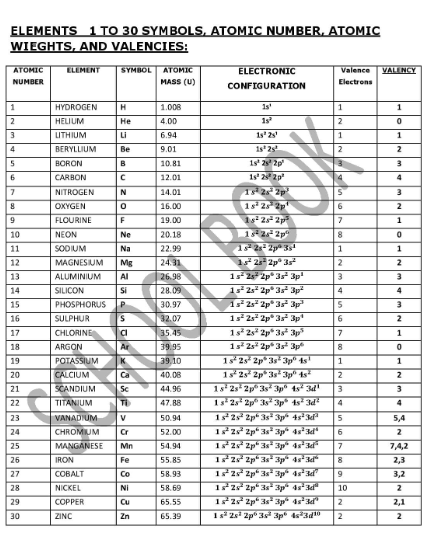
The question is asking for information related to the elements from 1 to 30, including their atomic numbers, symbols, atomic masses, electronic configurations, valence electrons, and valency.
Answer
Properties of elements 1-30 shown: atomic number, symbol, mass, electronic configuration, valence electrons, valency.
The table displays properties of elements 1 to 30, including atomic number, symbol, atomic mass, electronic configuration, valence electrons, and valency.
Answer for screen readers
The table displays properties of elements 1 to 30, including atomic number, symbol, atomic mass, electronic configuration, valence electrons, and valency.
More Information
Each element has unique properties that determine its chemical behavior. Atomic number identifies the number of protons in the nucleus. Atomic mass represents the average mass of an element's isotopes. The electronic configuration indicates how electrons are distributed in an atom.
Tips
A common mistake is confusing atomic mass with atomic number. Remember, atomic number refers to protons, while atomic mass is an average weight considering isotopes.
Sources
AI-generated content may contain errors. Please verify critical information