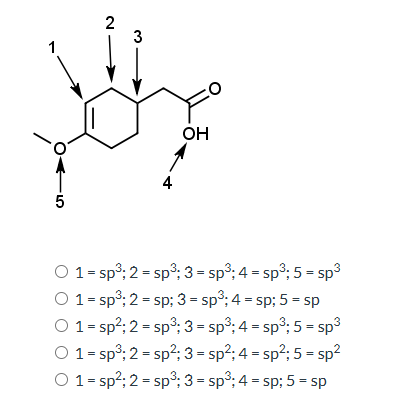
What are the hybridization states of the carbon atoms labeled 1 to 5 in the chemical structure?
Understand the Problem
The question is asking to determine the hybridization states of the carbon atoms in the given chemical structure. The answer choices provide different assignments of hybridization states to those specific carbon atoms labeled in the structure.
Answer
1 = sp2, 2 = sp2, 3 = sp3, 4 = sp2, 5 = sp2
The hybridization states are: 1 = sp2, 2 = sp2, 3 = sp3, 4 = sp2, 5 = sp2.
Answer for screen readers
The hybridization states are: 1 = sp2, 2 = sp2, 3 = sp3, 4 = sp2, 5 = sp2.
More Information
Carbon atom 1 and 2 are in a double bond and part of an aromatic ring, taking sp2 hybridization. Carbon 3 is bound to three atoms and has one lone pair, taking sp3 hybridization. Carbon 4 is part of a double bond and carboxyl group, hence sp2. Carbon 5 is also part of a carbon-oxygen double bond, taking sp2 hybridization.
Tips
A common mistake is not counting the total number of bonds and lone pairs correctly when determining hybridization.
Sources
- Finding the hybridization of atoms in organic molecules - khanacademy.org
- Hybridization of Carbon - Molecular Geometry and Bond Angles - byjus.com
- 1.8: Hybridization - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information