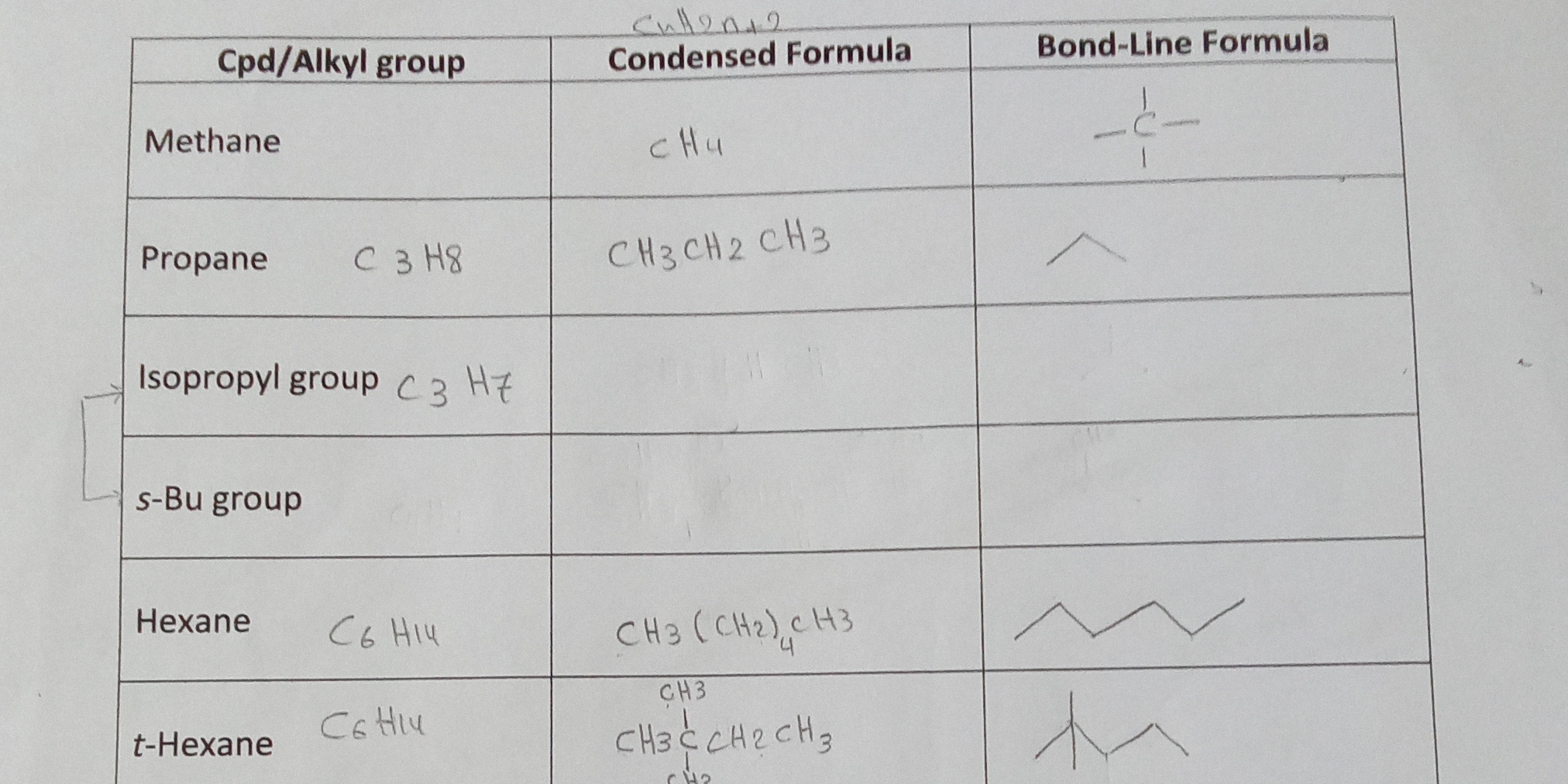
What are the condensed formulas and bond-line formulas for the following alkyl groups: Methane, Propane, Isopropyl group, s-Bu group, Hexane, and t-Hexane?
Understand the Problem
The question is about identifying organic compounds and their respective condensed and bond-line formulas, pertaining to alkyl groups such as methane, propane, isopropyl, and others.
Answer
Methane: CH4 (C), Propane: C3H8 (2 lines), Isopropyl: C3H7 ((CH3)2CH-), s-Bu: C4H9 ((CH3)2CHCH2-), Hexane: C6H14 (zigzag), t-Hexane: C6H13 ((CH3)3CCH2-).
The condensed and bond-line formulas are: Methane: CH4 (C), Propane: C3H8 (2 lines), Isopropyl group: C3H7 ((CH3)2CH-), s-Bu group: C4H9 ((CH3)2CHCH2-), Hexane: C6H14 (zigzag), t-Hexane: C6H13 ((CH3)3CCH2-).
Answer for screen readers
The condensed and bond-line formulas are: Methane: CH4 (C), Propane: C3H8 (2 lines), Isopropyl group: C3H7 ((CH3)2CH-), s-Bu group: C4H9 ((CH3)2CHCH2-), Hexane: C6H14 (zigzag), t-Hexane: C6H13 ((CH3)3CCH2-).
More Information
Alkyl groups are derived from alkanes by removing a hydrogen atom, which is indicated by changing the suffix from -ane to -yl.
Tips
Ensure the correct number of hydrogen atoms is removed for alkyl groups. For bond-line formulas, remember each vertex and endpoint represents a carbon atom.
Sources
- 3.3: Alkyl Groups - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information