What are indicators, and how do they change color in the presence of acids and bases? How can you prepare a natural indicator using red cabbage or china rose petals?
Understand the Problem
The question is asking for a summary of the information provided about indicators and their color changes in response to acids and bases, as well as the preparation of a natural indicator using red cabbage or china rose petals.
Answer
Indicators change color with acids or bases. Red cabbage and china rose petals can be used as natural indicators.
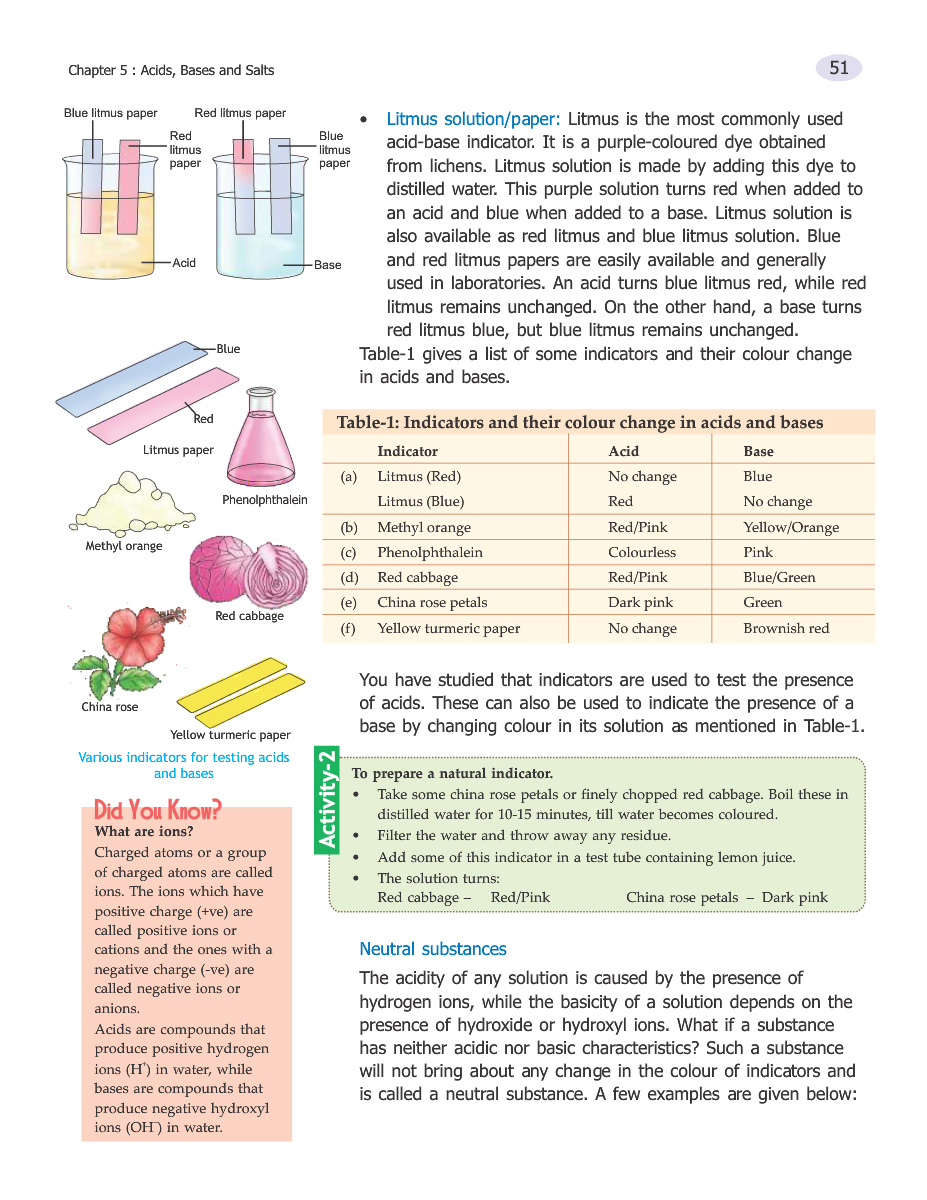
Indicators are substances that change color when exposed to acids or bases. Red cabbage turns red/pink with acids and blue/green with bases. China rose petals turn dark pink with acids and green with bases. Prepare a natural indicator by boiling red cabbage or china rose petals in water.
Answer for screen readers
Indicators are substances that change color when exposed to acids or bases. Red cabbage turns red/pink with acids and blue/green with bases. China rose petals turn dark pink with acids and green with bases. Prepare a natural indicator by boiling red cabbage or china rose petals in water.
More Information
Indicators like litmus, red cabbage, and china rose are useful in identifying the acidic or basic nature of a solution. Preparing natural indicators can be an interesting hands-on activity in learning chemistry concepts.
Tips
A common mistake is not preparing the natural indicator solution by boiling the plant material long enough to extract sufficient pigment.
Sources
AI-generated content may contain errors. Please verify critical information