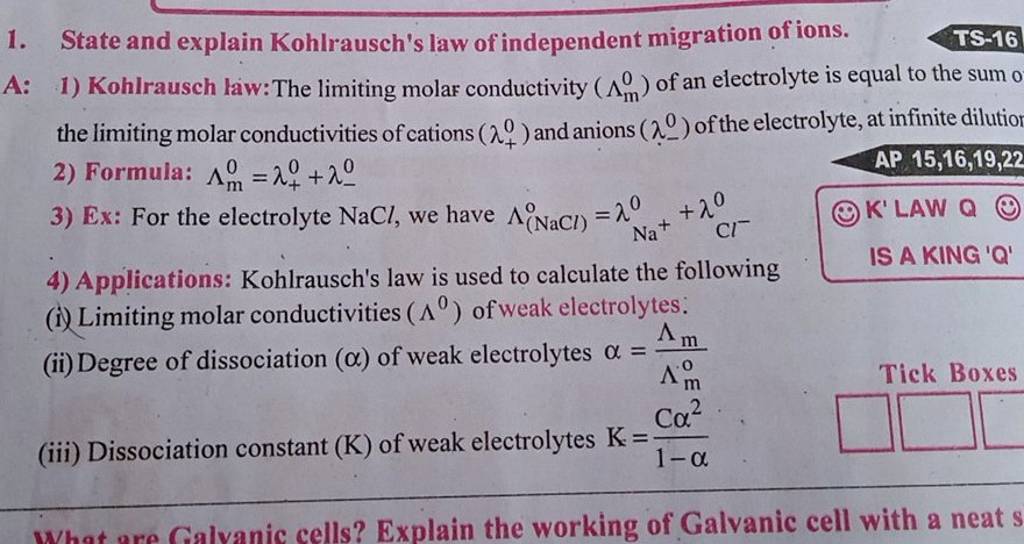
State and explain Kohlrausch's law of independent migration of ions.
Understand the Problem
The question is asking to state and explain Kohlrausch's law of independent migration of ions, which involves defining the law, providing the formula related to it, giving an example using the electrolyte NaCl, and discussing its applications in calculating limiting molar conductivities, degree of dissociation, and dissociation constant of weak electrolytes.
Answer
Kohlrausch's law states that at infinite dilution, each ion's contribution to molar conductivity is independent.
Kohlrausch's law states that the limiting molar conductivity of an electrolyte at infinite dilution is the sum of the individual ionic conductivities of the ions. At infinite dilution, each ion independently contributes to the total conductivity, irrespective of its co-ion.
Answer for screen readers
Kohlrausch's law states that the limiting molar conductivity of an electrolyte at infinite dilution is the sum of the individual ionic conductivities of the ions. At infinite dilution, each ion independently contributes to the total conductivity, irrespective of its co-ion.
More Information
Kohlrausch's law is instrumental in calculating various properties related to electrolytes, like the degree of dissociation and dissociation constants, especially for weak electrolytes. It simplifies the study of ionic conductivities by allowing the separation of contributions from individual ions.
Tips
A common mistake is forgetting that the law applies only at infinite dilution, where ion interactions are negligible.
Sources
- Kohlrausch's law of independent migration - Byjus - byjus.com
- State and explain Kohlrausch's law - Toppr - toppr.com
AI-generated content may contain errors. Please verify critical information