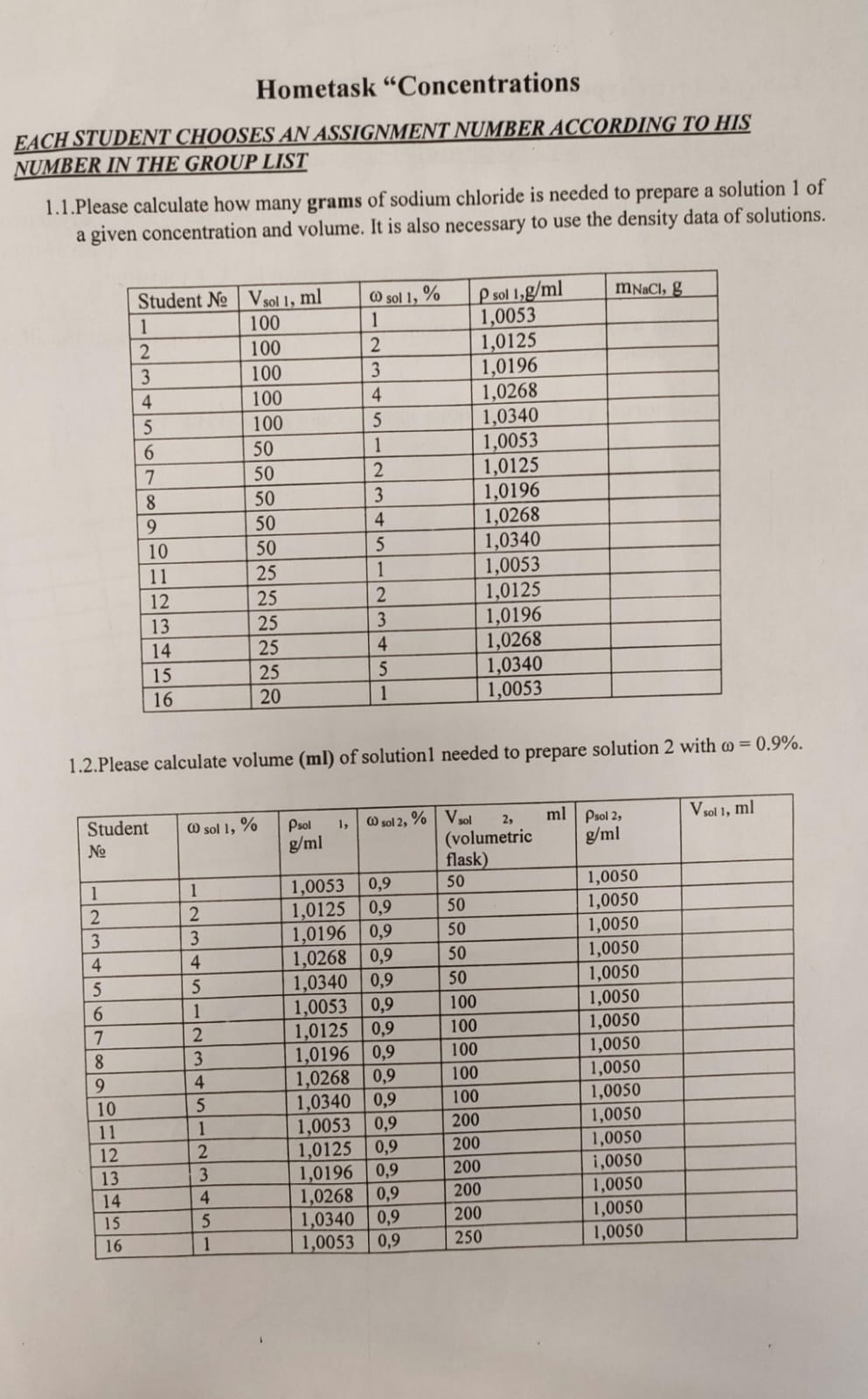
Please calculate how many grams of sodium chloride is needed to prepare a solution of a given concentration and volume. It is also necessary to calculate the volume of solution nee... Please calculate how many grams of sodium chloride is needed to prepare a solution of a given concentration and volume. It is also necessary to calculate the volume of solution needed to prepare another solution with a different concentration.
Understand the Problem
The question is asking to calculate the grams of sodium chloride required for a certain volume and concentration of a solution, as well as the volume needed for a second solution with a specified concentration. This involves using given density data to perform the calculations.
Answer
The mass of sodium chloride needed is $1.0053 \, g$ and the required volume of solution 1 is approximately $111.9 \, ml$.
Answer for screen readers
For Student 1, the mass of sodium chloride needed is $m_{NaCl} = 1.0053 , g$. The volume of solution 1 needed to prepare solution 2 is approximately $V_{sol2} \approx 111.9 , ml$.
Steps to Solve
- Identify Key Variables We need the following information from the table for Student 1:
- Volume of solution ($V_{sol1}$) = 100 ml
- Weight percent of solution ($\omega_{sol1}$) = 1%
- Density of solution ($\rho_{sol1}$) = 1.0053 g/ml
-
Calculate the Mass of Sodium Chloride The mass of sodium chloride ($m_{NaCl}$) can be determined using the formula: $$ m_{NaCl} = V_{sol1} \times \frac{\omega_{sol1}}{100} \times \rho_{sol1} $$ Substituting the known values: $$ m_{NaCl} = 100 , \text{ml} \times \frac{1}{100} \times 1.0053 , \text{g/ml} $$
-
Performing the Calculation Now we perform the calculation: $$ m_{NaCl} = 100 \times 0.01 \times 1.0053 = 1.0053 , \text{g} $$
-
Volume Required for Second Solution Next, we calculate the volume of solution 1 needed to prepare solution 2 with $\omega_{sol2} = 0.9%$ using the formula: $$ V_{sol2} = \frac{m_{NaCl}}{\frac{\omega_{sol2}}{100} \times \rho_{sol2}} $$ Where $\rho_{sol2} = 1.0050 , \text{g/ml}$ (from the table). Substituting values: $$ V_{sol2} = \frac{1.0053 , \text{g}}{\frac{0.9}{100} \times 1.0050 , \text{g/ml}} $$
-
Calculating the Volume for Solution 2 Scheduling the calculation: $$ V_{sol2} = \frac{1.0053}{0.009045} \approx 111.9 , \text{ml} $$
For Student 1, the mass of sodium chloride needed is $m_{NaCl} = 1.0053 , g$. The volume of solution 1 needed to prepare solution 2 is approximately $V_{sol2} \approx 111.9 , ml$.
More Information
In this problem, we used the concentration, volume, and density of sodium chloride solutions to calculate the amount of solute required. This method is important in chemistry for preparing solutions of specific concentrations.
Tips
- Forgetting to convert the percentage to a decimal before multiplication.
- Misapplying the density when calculating masses or volumes.
- Not double-checking units for consistency.
AI-generated content may contain errors. Please verify critical information