Understand the Problem
The question appears to be related to an experiment involving particles, scattering, and atomic structure. It likely seeks to understand the outcomes and implications of particle interactions with a gold foil, maybe related to Rutherford's gold foil experiment.
Answer
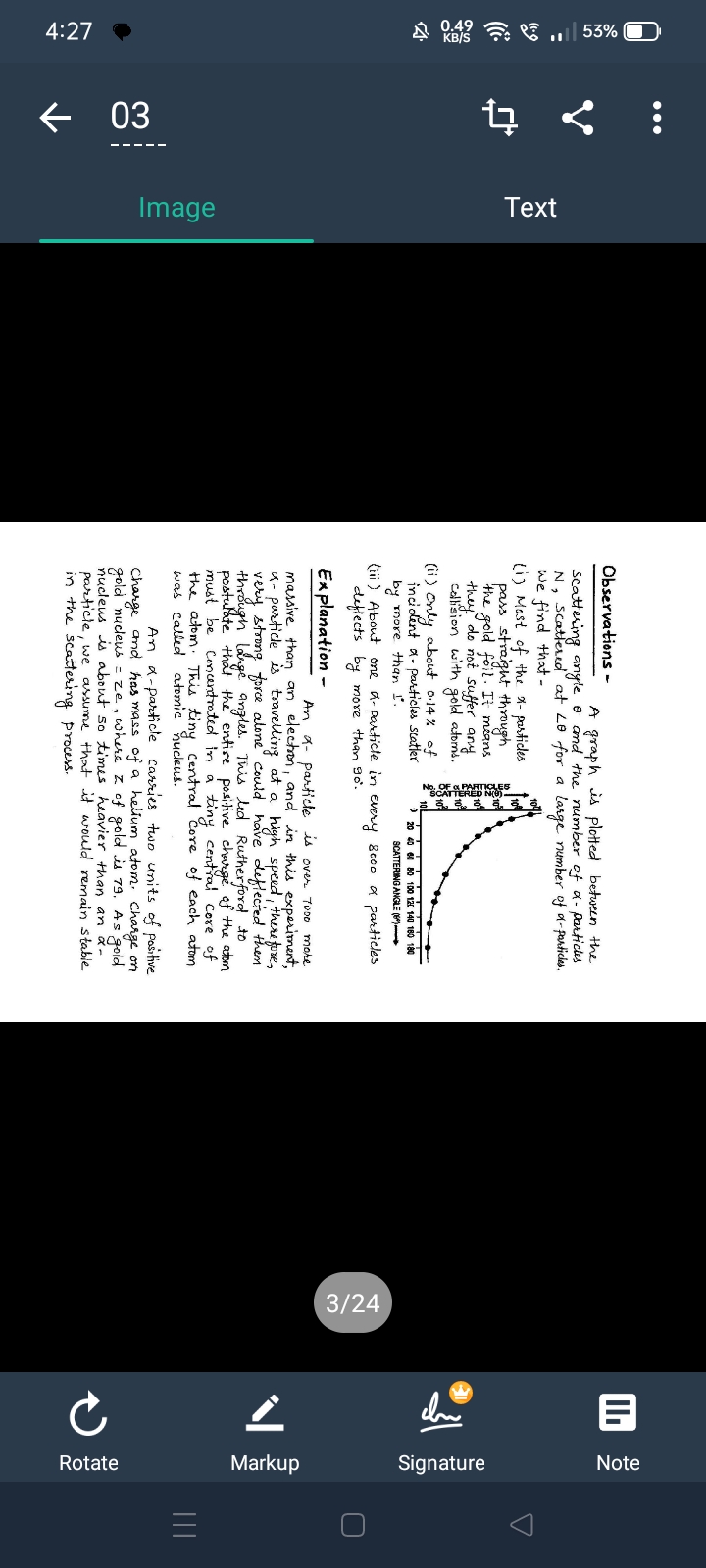
Rutherford's gold foil experiment.
The concept likely being discussed is Rutherford's gold foil experiment, which demonstrated that the atom consists of a small, dense nucleus. Alpha particles occasionally scatter at large angles when they strike the nucleus.
Answer for screen readers
The concept likely being discussed is Rutherford's gold foil experiment, which demonstrated that the atom consists of a small, dense nucleus. Alpha particles occasionally scatter at large angles when they strike the nucleus.
More Information
In Rutherford's experiment, most alpha particles passed through the foil, but some deflected at large angles, suggesting a dense nucleus. This overturned the plum pudding model of the atom.
Tips
Ensure the interpretation of scattering angles and the role of the nucleus is clear.
AI-generated content may contain errors. Please verify critical information