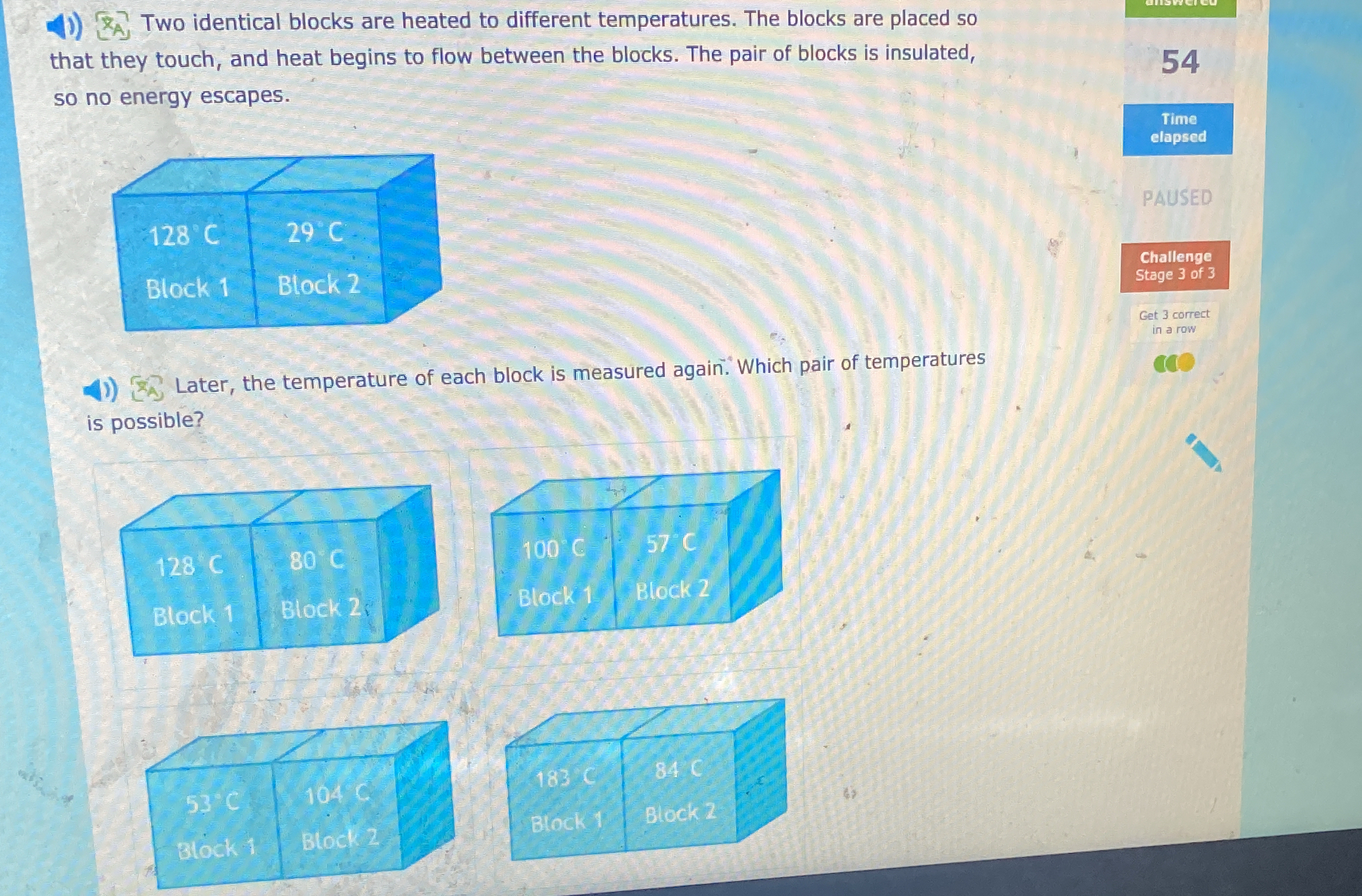
Later, the temperature of each block is measured again. Which pair of temperatures is possible?
Understand the Problem
The question is asking about the equilibrium temperature reached by two identical blocks initially at different temperatures after being insulated from the environment. The user needs to determine which of the given pairs of temperatures for the blocks is possible after they have exchanged heat.
Answer
100°C Block 1, 57°C Block 2
The final answer is 100°C for Block 1 and 57°C for Block 2.
Answer for screen readers
The final answer is 100°C for Block 1 and 57°C for Block 2.
More Information
The blocks are insulated, so no energy escapes. They will reach a thermal equilibrium where the temperatures are closer together but not equal, as no energy is lost to the environment.
Tips
A common mistake is assuming the blocks will reach the same temperature. Since they are insulated and no energy escapes, the temperatures will equalize between the initial values.
Sources
AI-generated content may contain errors. Please verify critical information