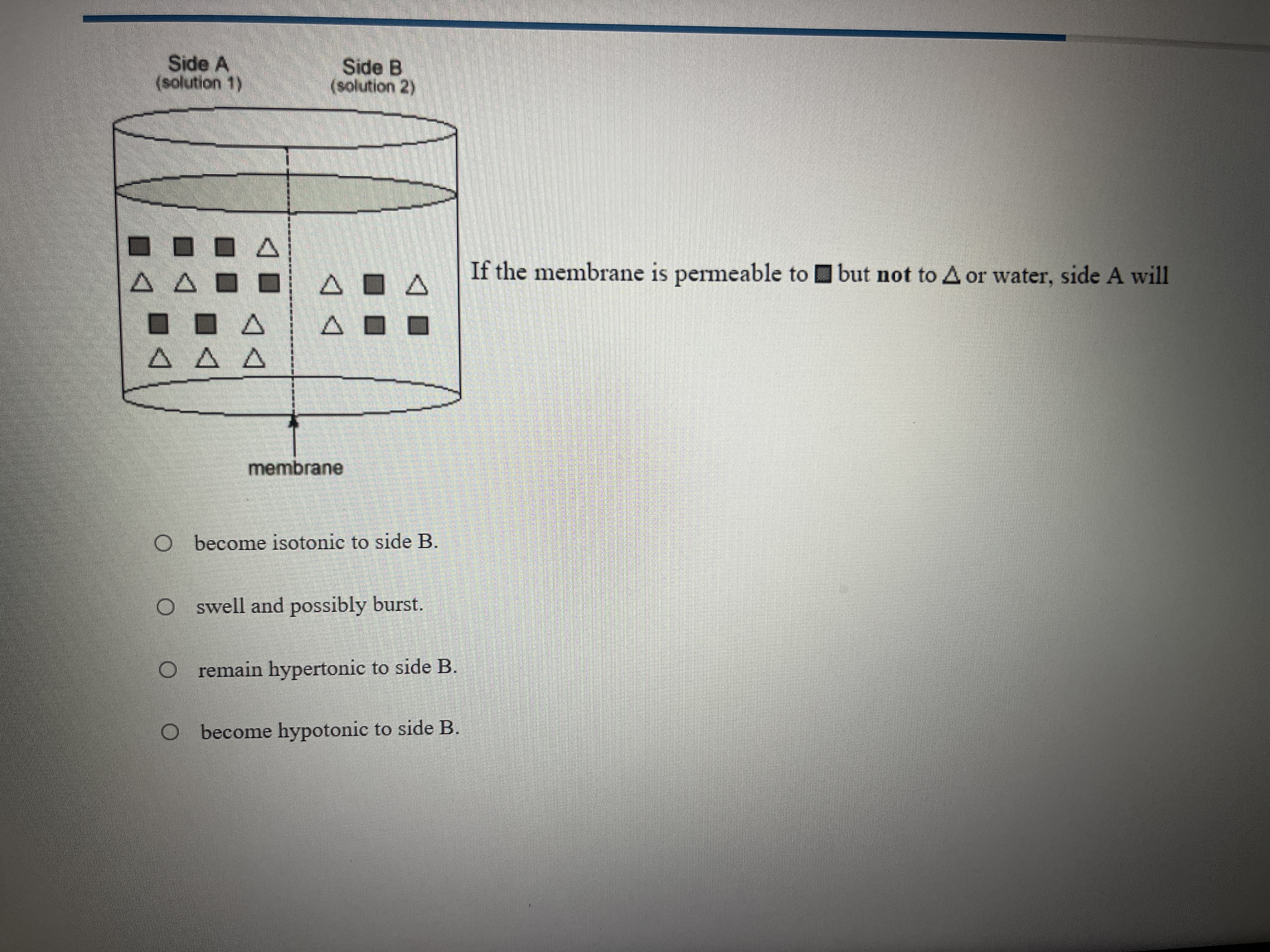
If the membrane is permeable to certain solutes but not to others or water, what will happen to side A?
Understand the Problem
The question is asking about the behavior of solution A in relation to solution B when a selectively permeable membrane is involved. It addresses the concept of osmosis and the effects of solute and solvent permeability on the concentrations on either side of the membrane.
Answer
Side A will become isotonic to side B.
Side A will become isotonic to side B.
Answer for screen readers
Side A will become isotonic to side B.
More Information
Since squares can move across the membrane, they will eventually equalize in concentration, making the solutions isotonic with respect to this solute. However, since water and triangles cannot move, the overall tonicity concerning all solutes remains unchanged.
Tips
Confusion often arises when considering solute-specific permeability. It's important to differentiate between permeable and impermeable solutes for determining overall tonicity changes.
AI-generated content may contain errors. Please verify critical information