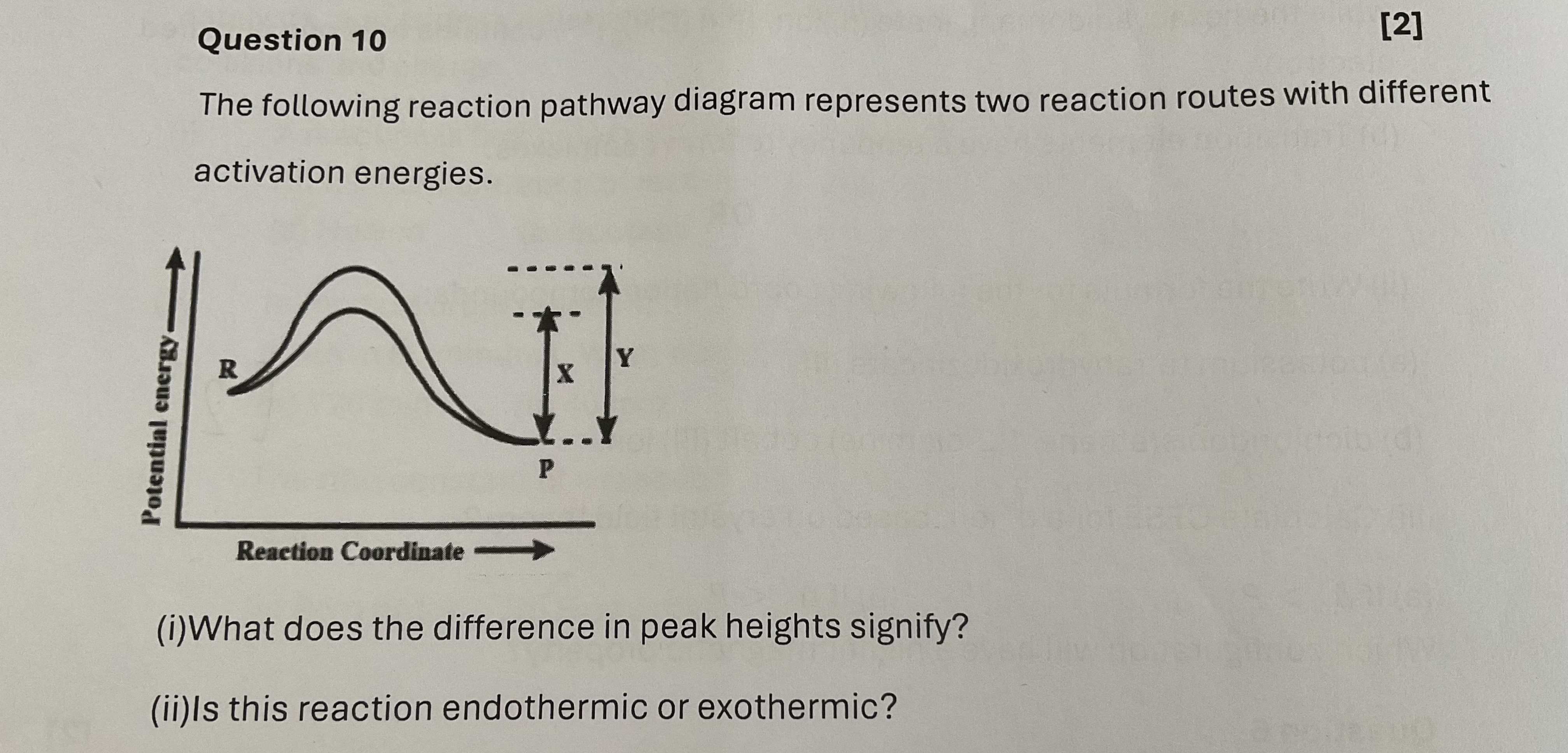
(i) What does the difference in peak heights signify? (ii) Is this reaction endothermic or exothermic?
Understand the Problem
The question is asking about a reaction pathway diagram that represents two reaction routes with different activation energies. Specifically, it seeks to understand the significance of the difference in peak heights in the diagram and whether the reaction is endothermic or exothermic.
Answer
(i) Different activation energies. (ii) Exothermic reaction.
(i) The difference in peak heights signifies different activation energies of the reactions; higher peaks represent higher activation energy. (ii) Since the potential energy decreases from R to P, the reaction is exothermic.
Answer for screen readers
(i) The difference in peak heights signifies different activation energies of the reactions; higher peaks represent higher activation energy. (ii) Since the potential energy decreases from R to P, the reaction is exothermic.
More Information
Activation energy is the minimum energy required for a reaction to occur. In energy diagrams, reaction direction (exothermic or endothermic) is inferred from the changes in energy levels of reactants and products.
Tips
A common mistake is confusing activation energy with the total energy change in the reaction.
Sources
- Endothermic vs. exothermic reactions (article) - Khan Academy - khanacademy.org
- Representing endothermic and exothermic processes using energy diagrams - Khan Academy - khanacademy.org
AI-generated content may contain errors. Please verify critical information