How many neutrons can be found in an atom of Na (Sodium)?
Understand the Problem
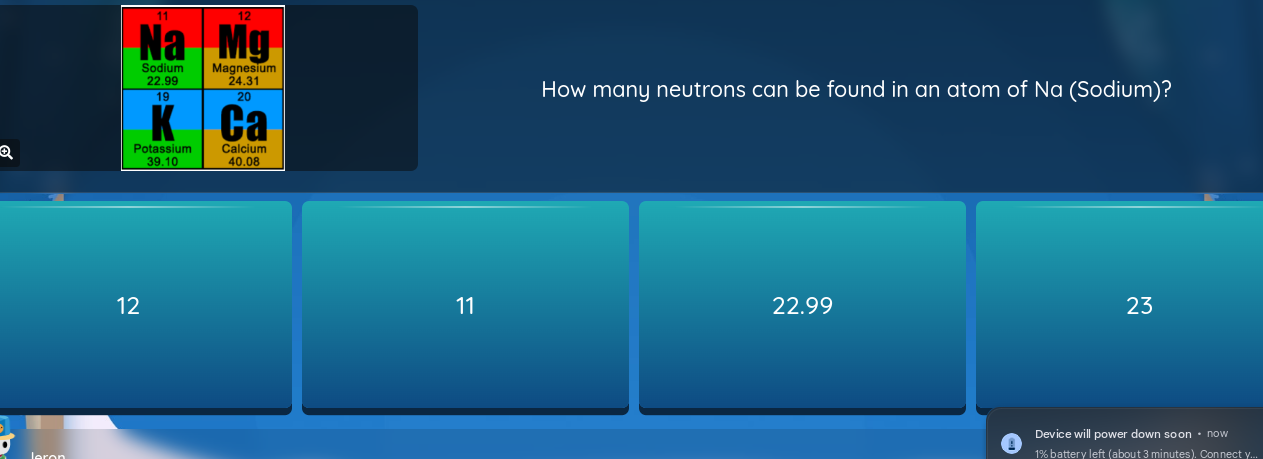
The question is asking to determine the number of neutrons in an atom of sodium (Na). To find this, we need to subtract the atomic number of sodium (which is 11) from its atomic mass (approximately 23).
Answer
The number of neutrons in an atom of sodium is $12$.
Answer for screen readers
The number of neutrons in an atom of sodium (Na) is $12$.
Steps to Solve
-
Identify the atomic number and atomic mass of sodium The atomic number of sodium (Na) is 11. The atomic mass is approximately 23.
-
Calculate the number of neutrons To find the number of neutrons, use the formula: $$ \text{Neutrons} = \text{Atomic Mass} - \text{Atomic Number} $$
Substituting the values: $$ \text{Neutrons} = 23 - 11 $$
- Perform the subtraction Calculate the result: $$ \text{Neutrons} = 12 $$
The number of neutrons in an atom of sodium (Na) is $12$.
More Information
Sodium has 11 protons and 12 neutrons, making its atomic mass approximately 23. The number of neutrons can vary slightly in isotopes of sodium, but for the most common isotope, this is correct.
Tips
- Confusing atomic mass with atomic number: Remember that the atomic number represents the number of protons, while the atomic mass includes both protons and neutrons.
- Not rounding the atomic mass correctly: Ensure to use the correct rounded atomic mass value based on available data.
AI-generated content may contain errors. Please verify critical information