How many grams of H2O are present in 0.25 moles of it?
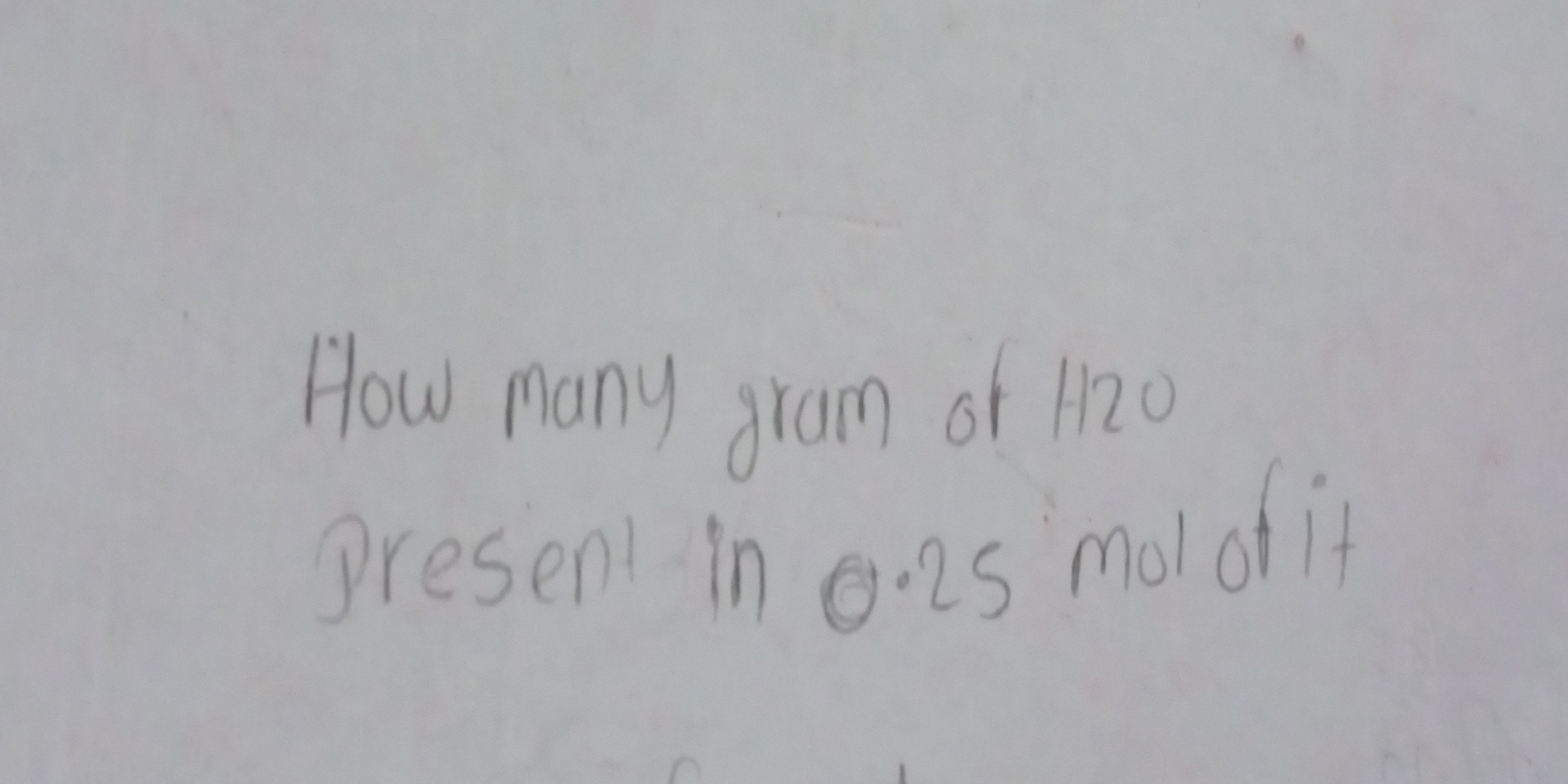
Understand the Problem
The question is asking for the mass of H2O (water) in grams that corresponds to a quantity of 0.25 moles. To solve this, we will need to use the molar mass of water, which is approximately 18.02 g/mol, and apply the formula: mass = moles × molar mass.
Answer
The mass of H2O is approximately 4.51 g.
Answer for screen readers
The mass of H2O present in 0.25 mol is approximately 4.51 g.
Steps to Solve
- Identify the molar mass of water (H2O)
The molar mass of water is approximately 18.02 g/mol. This value is essential for calculating the mass from the number of moles.
- Set up the formula for mass
Using the formula ( \text{mass} = \text{moles} \times \text{molar mass} ), we can now substitute the values.
- Calculate the mass
Substituting the given values into the formula:
$$ \text{mass} = 0.25 , \text{mol} \times 18.02 , \text{g/mol} $$
- Perform the arithmetic
Now, calculate the mass:
$$ \text{mass} = 0.25 \times 18.02 = 4.505 , \text{g} $$
The mass of H2O present in 0.25 mol is approximately 4.51 g.
More Information
This calculation shows how to convert moles of a substance into grams using its molar mass, which is a fundamental concept in chemistry related to stoichiometry.
Tips
- Not using the correct molar mass for water. Ensure to use 18.02 g/mol.
- Forgetting to multiply properly. Double-check arithmetic calculations to avoid mistakes.
AI-generated content may contain errors. Please verify critical information