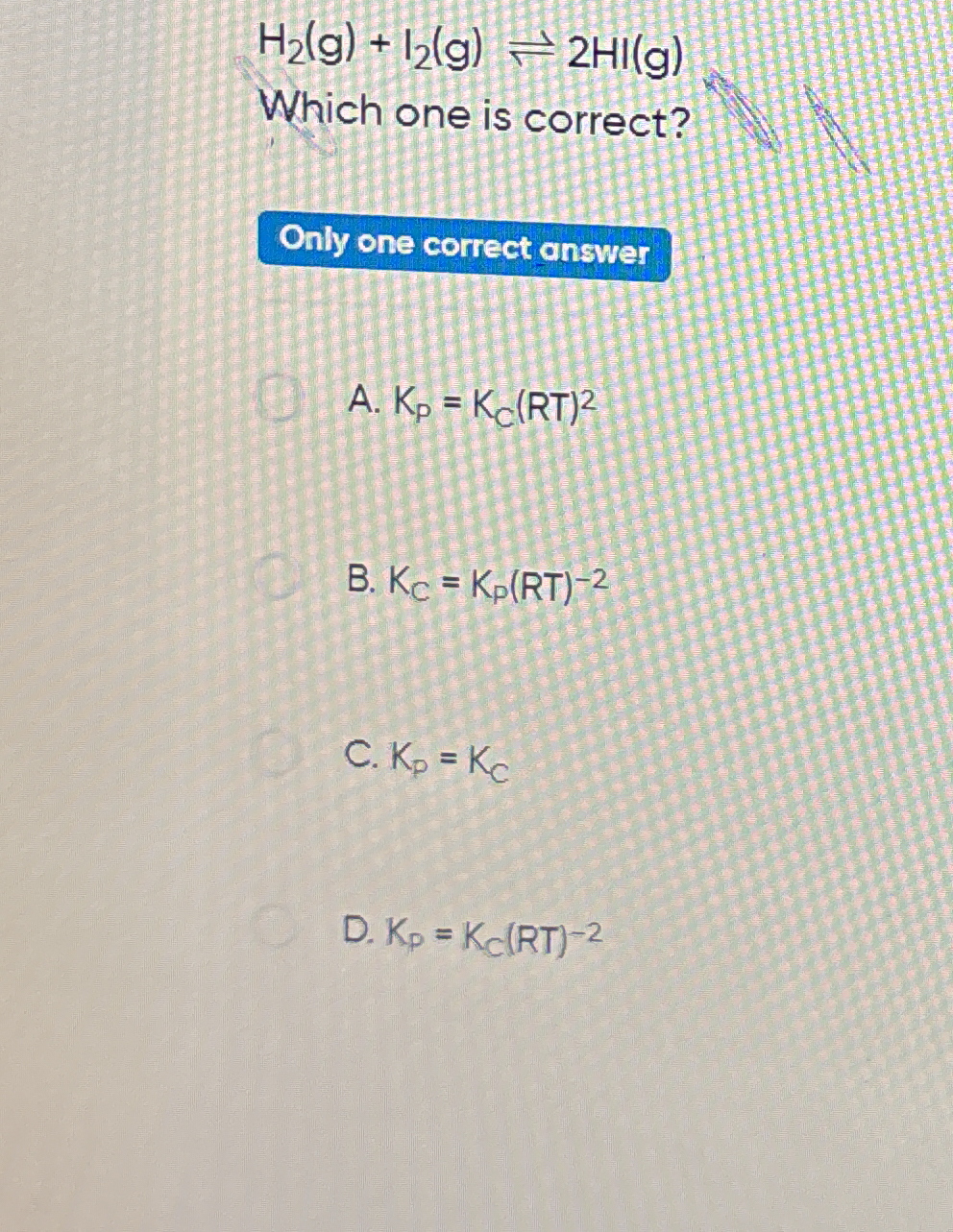
H2(g) + I2(g) ⇌ 2HI(g) Which one is correct?
Understand the Problem
The question is asking about the relationship between the equilibrium constants Kp and Kc for the given chemical reaction, and it provides multiple statements to evaluate which one is correct.
Answer
Kp = Kc
The correct answer is C: Kp = Kc.
Answer for screen readers
The correct answer is C: Kp = Kc.
More Information
For the reaction H2(g) + I2(g) ⇌ 2HI(g), Δn is 0 so Kp = Kc.
Tips
A common mistake is not calculating Δn correctly, leading to the wrong relationship between Kp and Kc.
Sources
AI-generated content may contain errors. Please verify critical information