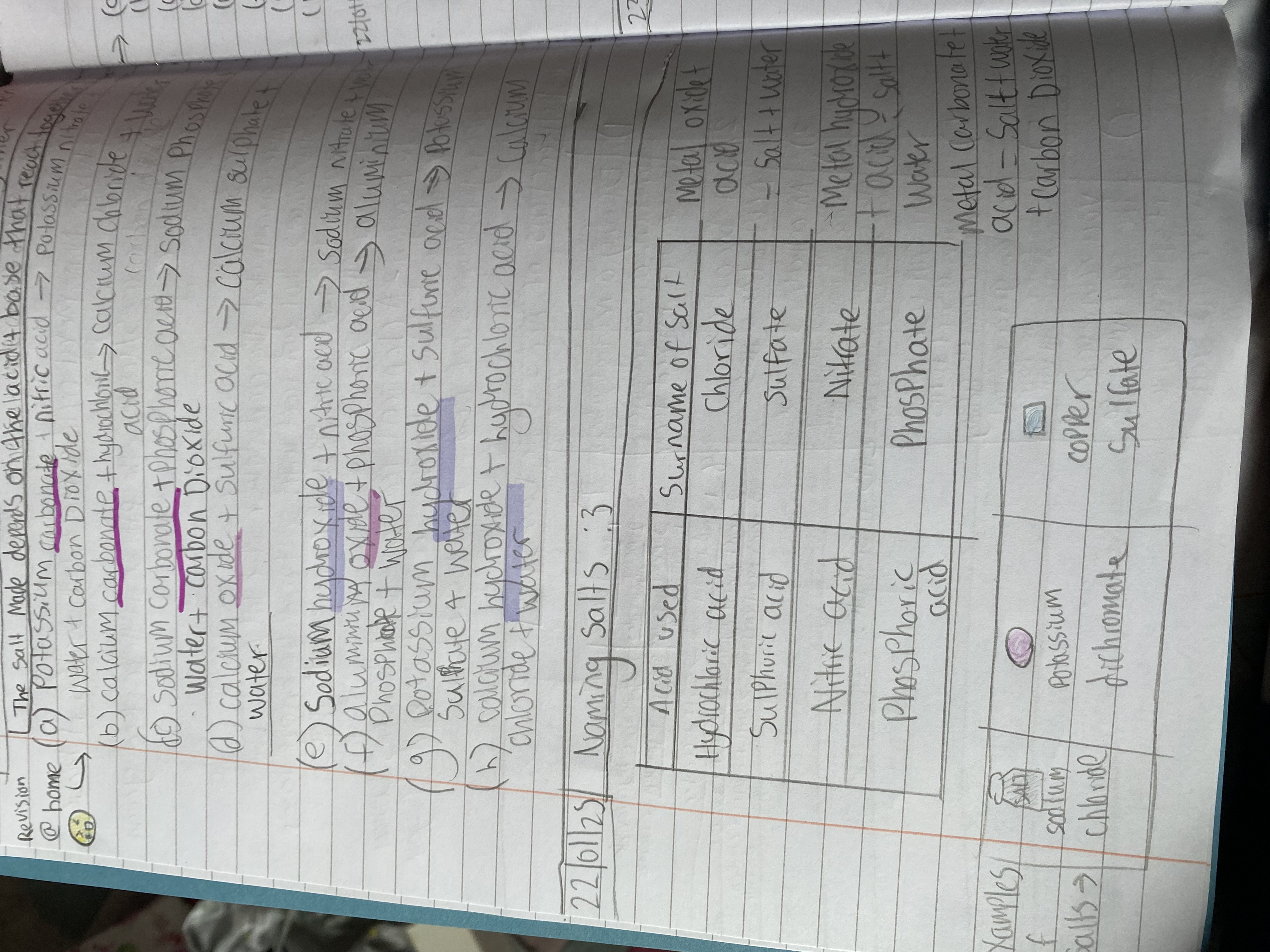
Explain how to name the salts produced from the reactions detailed, using the acids and bases listed.
Understand the Problem
The question is asking to understand the reactions involved in naming salts produced from different acids and bases. It seeks to clarify how various salts are formed from specific acids and bases, including examples of those salts.
Answer
Salt's name = cation (base) + anion (acid).
The salt name consists of two parts: the cation from the base and the anion from the acid. For example, sodium hydroxide (base) and hydrochloric acid (acid) form sodium chloride (salt).
Answer for screen readers
The salt name consists of two parts: the cation from the base and the anion from the acid. For example, sodium hydroxide (base) and hydrochloric acid (acid) form sodium chloride (salt).
More Information
Salts are typically formed when an acid reacts with a base, resulting in ionic compounds with diverse applications, including culinary (e.g., sodium chloride) and industrial (e.g., copper sulfate) uses.
Tips
A common mistake is mixing up the source of the cation and anion; remember the cation is from the base and the anion is from the acid.
Sources
- Naming salts - Acids, alkalis and salts - AQA Trilogy - BBC Bitesize - bbc.co.uk
- 4.10: Acids, Bases and Salts - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information