Exercice de chimie organique sur des peptides, des structures de molécules, des groupes fonctionnels et des configurations électroniques.
Understand the Problem
La question porte sur des exercices de chimie organique, incluant des structures de molécules, des groupes fonctionnels, et des concepts de configuration électronique et de projections de Newman.
Answer
Identify, draw, and analyze molecular structures, functional groups, bonds, and configurations according to given chemical information.
For each part:
-
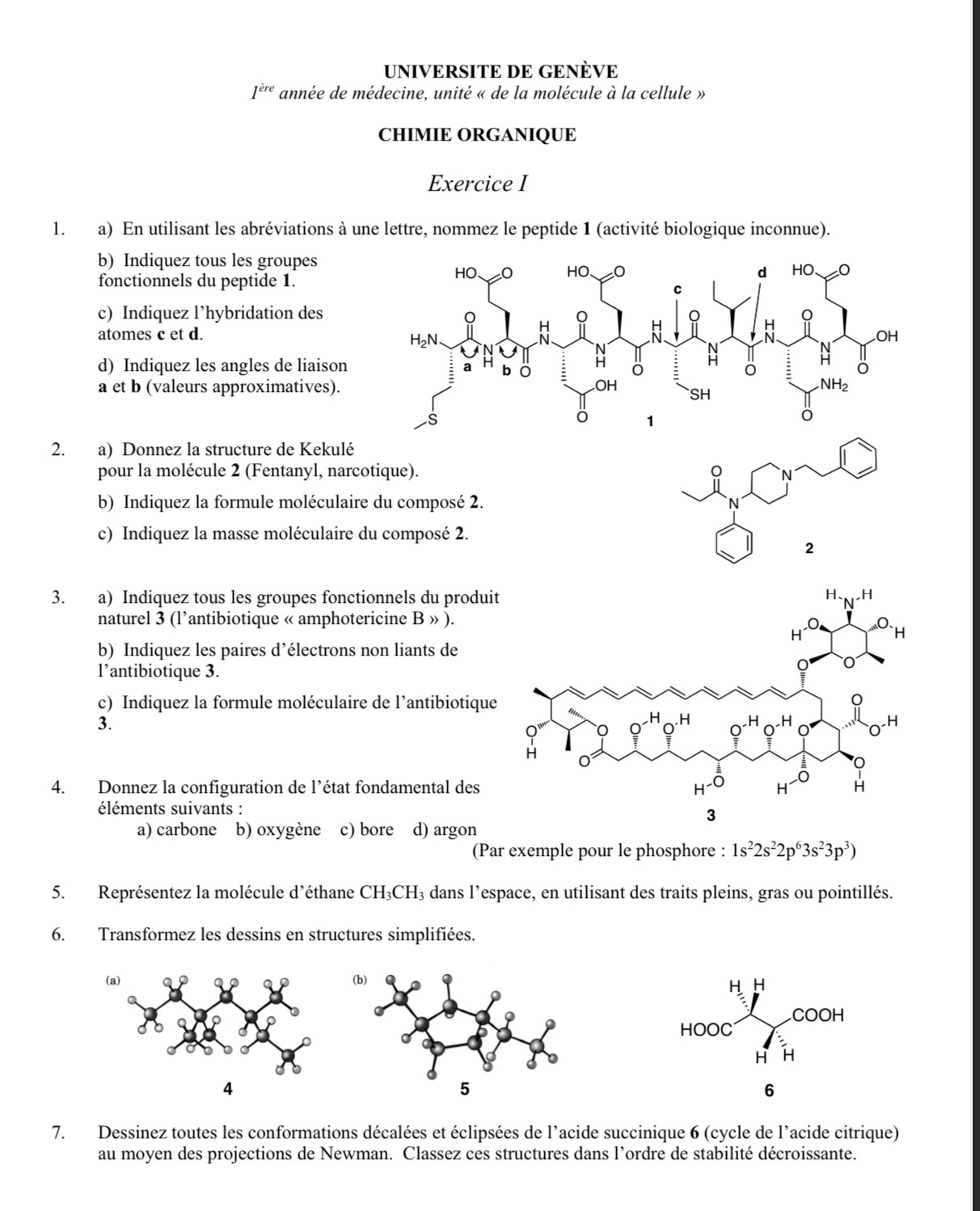
a) Use single letter codes to name peptide. b) Identify the amide and carboxyl groups, among others. c) Hybridization: likely sp2 or sp3 based on environment. d) Bond angles can range around 109.5° or 120° depending on hybridization.
-
a) Kekulé structures involve drawing all atoms and bonds for a molecule. b) Formulate C22H28N2O. c) Calculate based on atomic weights: ~336 g/mol.
-
a) Functional groups include lactone, double bonds, etc. b) Electron pairs: Oxygen lone pairs are notable. c) Formula likely around C47H73NO17.
-
a) Carbon: 1s² 2s² 2p². b) Oxygen: 1s² 2s² 2p⁴. c) Boron: 1s² 2s² 2p¹. d) Argon: 1s² 2s² 2p⁶ 3s² 3p⁶.
-
Use 3D representation conventions for ethane.
-
Simplified structure drawings involve skeletal formulas.
-
Project Newman-style conformations and rank from staggered (most stable) to eclipsed (least stable).
Answer for screen readers
For each part:
-
a) Use single letter codes to name peptide. b) Identify the amide and carboxyl groups, among others. c) Hybridization: likely sp2 or sp3 based on environment. d) Bond angles can range around 109.5° or 120° depending on hybridization.
-
a) Kekulé structures involve drawing all atoms and bonds for a molecule. b) Formulate C22H28N2O. c) Calculate based on atomic weights: ~336 g/mol.
-
a) Functional groups include lactone, double bonds, etc. b) Electron pairs: Oxygen lone pairs are notable. c) Formula likely around C47H73NO17.
-
a) Carbon: 1s² 2s² 2p². b) Oxygen: 1s² 2s² 2p⁴. c) Boron: 1s² 2s² 2p¹. d) Argon: 1s² 2s² 2p⁶ 3s² 3p⁶.
-
Use 3D representation conventions for ethane.
-
Simplified structure drawings involve skeletal formulas.
-
Project Newman-style conformations and rank from staggered (most stable) to eclipsed (least stable).
More Information
This exercise covers fundamental organic chemistry concepts including peptide structure, functional groups, molecular geometry, and electronic configurations. Such knowledge is critical for understanding chemical reactivity and synthesis.
Tips
Common mistakes include incorrect hybridization assignment and misunderstanding molecular geometry. Pay attention to bond angle approximations.
Sources
- Exercices résolus de chimie organique - Numilog - excerpts.numilog.com
- Les acides aminés et la synthèse peptidique | CultureSciences-Chimie - culturesciences.chimie.ens.fr
- 20.E : Chimie organique (exercices) - Global - LibreTexts - query.libretexts.org
AI-generated content may contain errors. Please verify critical information