Describe how Mike makes crystals of pure zinc chloride from the mixture.
Understand the Problem
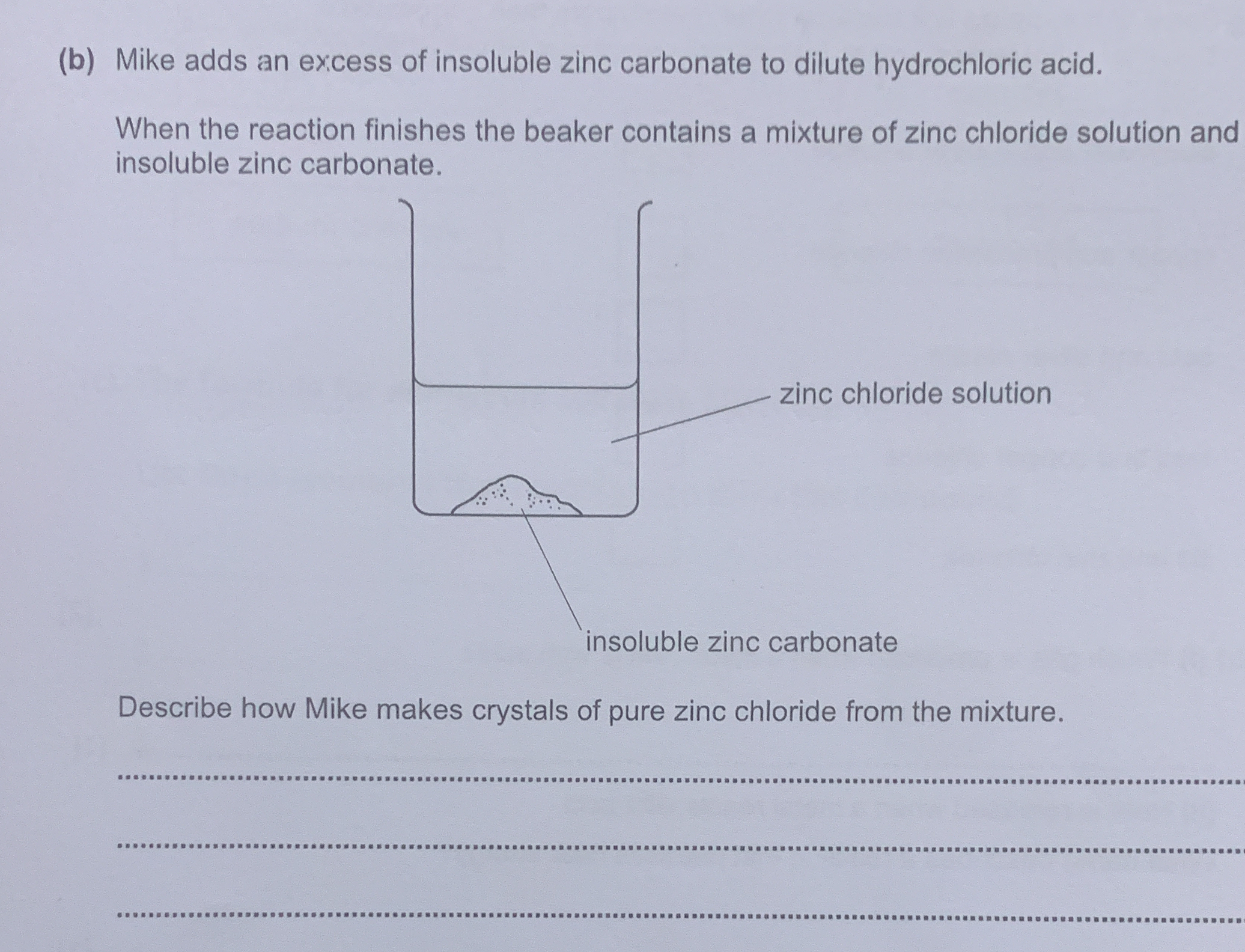
The question is asking for a step-by-step description of how Mike can obtain pure zinc chloride crystals from a mixture that contains both zinc chloride solution and insoluble zinc carbonate. The process likely involves filtering, evaporating, and crystallizing the zinc chloride from the solution.
Answer
Filter the solution, then evaporate to crystallize zinc chloride.
To make pure zinc chloride crystals, filter the mixture to remove excess zinc carbonate, then evaporate the zinc chloride solution to crystallize the zinc chloride.
Answer for screen readers
To make pure zinc chloride crystals, filter the mixture to remove excess zinc carbonate, then evaporate the zinc chloride solution to crystallize the zinc chloride.
More Information
The process involves a reaction between zinc carbonate and hydrochloric acid, followed by filtration and evaporation to form crystals.
Tips
Ensure complete filtration to remove impurities before evaporation.
Sources
- How to safely make zinc chloride - Quora - quora.com
AI-generated content may contain errors. Please verify critical information