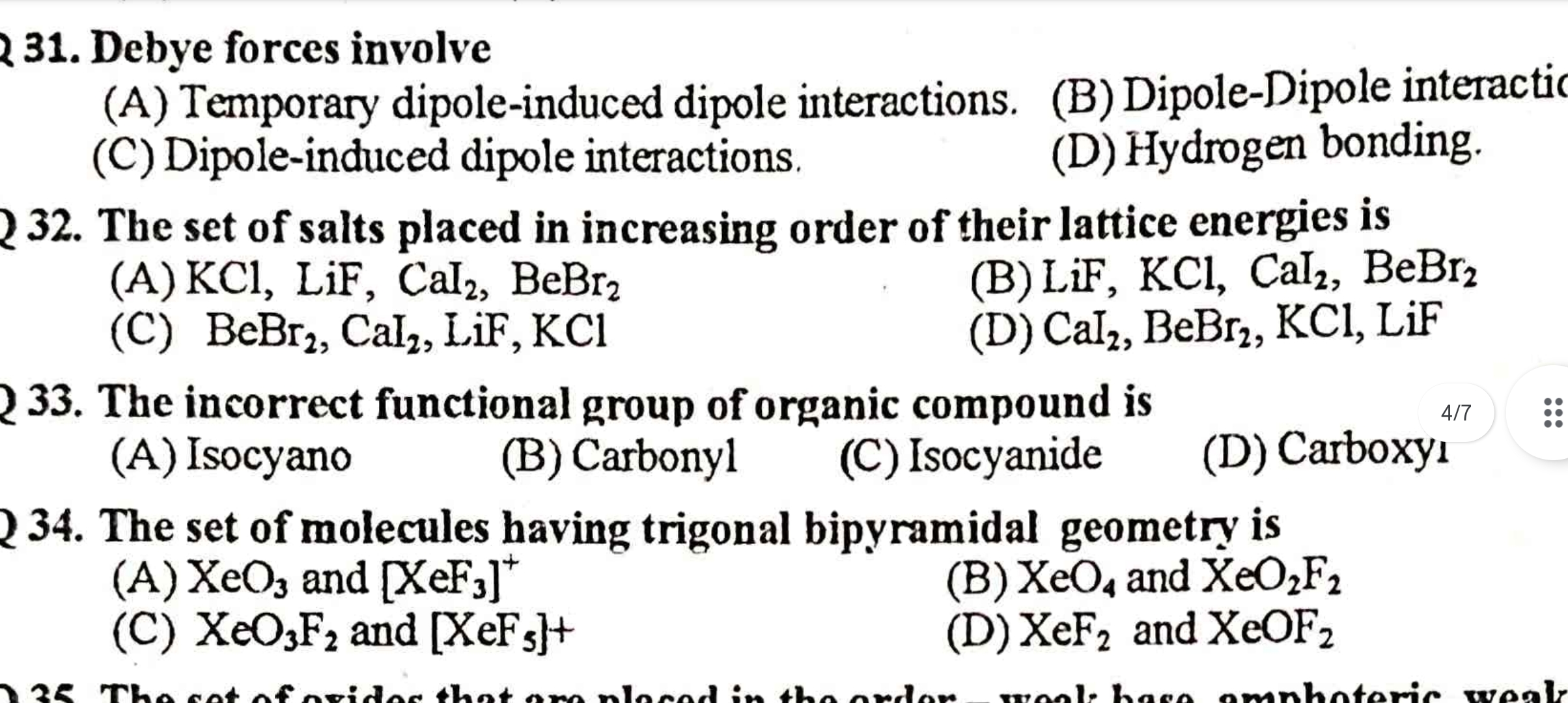
Debye forces involve: (A) Temporary dipole-induced dipole interactions, (B) Dipole-Dipole interactions, (C) Dipole-induced dipole interactions, (D) Hydrogen bonding. The set of sal... Debye forces involve: (A) Temporary dipole-induced dipole interactions, (B) Dipole-Dipole interactions, (C) Dipole-induced dipole interactions, (D) Hydrogen bonding. The set of salts placed in increasing order of their lattice energies is: (A) KCl, LiF, CaI2, BeBr2, (B) LiF, KCl, CaI2, BeBr2, (C) BeBr2, CaI2, LiF, KCl. The incorrect functional group of organic compound is: (A) Isocyan, (B) Carbonyl, (C) Isocyanide, (D) Carboxyl. The set of molecules having trigonal bipyramidal geometry is: (A) XeO3 and [XeF3]+, (B) XeO4 and XeO2F2, (C) XeO3F2 and [XeF5]+.
Understand the Problem
The questions are asking about concepts in chemistry, specifically regarding Debye forces, lattice energies, functional groups in organic compounds, and molecular geometry. Each question presents multiple choice answers related to these concepts.
Answer
31) C, 32) D, 33) A, 34) C
The answers are: 31) C, 32) D, 33) A, 34) C.
Answer for screen readers
The answers are: 31) C, 32) D, 33) A, 34) C.
More Information
Debye forces are dipole-induced dipole interactions. Lattice energy order depends on ionic size and charge. 'Isocyano' is not a typical functional group name. Trigonal bipyramidal geometry involves five electron regions around the central atom.
Tips
Ensure understanding of intermolecular forces. Avoid confusing functional groups with less common terms.
Sources
- 12.6: Intermolecular Forces- Dispersion, Dipole–Dipole, Hydrogen ... - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information