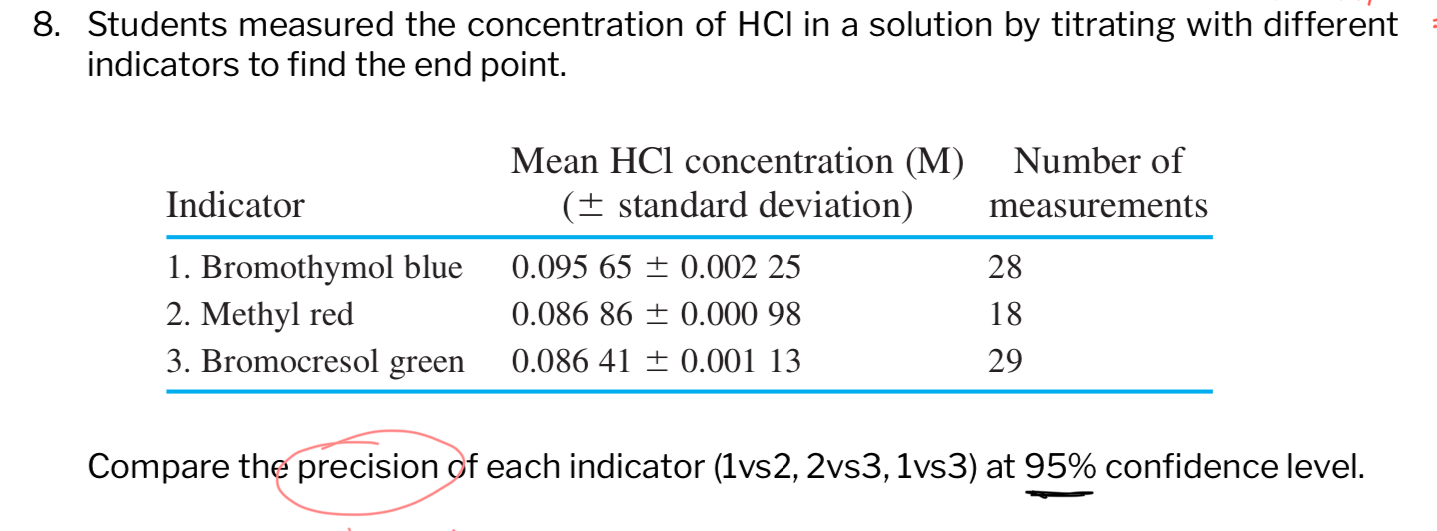
Compare the precision of each indicator (1 vs 2, 2 vs 3, 1 vs 3) at a 95% confidence level, given the following information: Indicator 1 (Bromothymol blue): Mean = 0.09565 M, Stan... Compare the precision of each indicator (1 vs 2, 2 vs 3, 1 vs 3) at a 95% confidence level, given the following information: Indicator 1 (Bromothymol blue): Mean = 0.09565 M, Standard deviation = 0.00225 M, n = 28 Indicator 2 (Methyl red): Mean = 0.08686 M, Standard deviation = 0.00098 M, n = 18 Indicator 3 (Bromocresol green): Mean = 0.08641 M, Standard deviation = 0.00113 M, n = 29
Understand the Problem
The question asks to compare the precision of three different indicators (Bromothymol blue, Methyl red, and Bromocresol green) used in the titration of HCl, based on the provided mean concentrations, standard deviations, and number of measurements. Specifically, you need to perform a pairwise comparison (1 vs 2, 2 vs 3, and 1 vs 3) at a 95% confidence level to determine which indicator yields more precise results.
Answer
Precision is determined by comparing standard deviations. Lower standard deviation indicates higher precision. Therefore, Methyl red (Indicator 2) is the most precise, followed by Bromocresol green (Indicator 3), and then Bromothymol blue (Indicator 1).
To compare the precision of the indicators, compare their standard deviations. At a 95% confidence level:
- 1 vs 2: Indicator 2 (Methyl red, SD = 0.00098 M) is more precise than Indicator 1 (Bromothymol blue, SD = 0.00225 M).
- 2 vs 3: Indicator 2 (Methyl red, SD = 0.00098 M) is more precise than Indicator 3 (Bromocresol green, SD = 0.00113 M).
- 1 vs 3: Indicator 3 (Bromocresol green, SD = 0.00113 M) is more precise than Indicator 1 (Bromothymol blue, SD = 0.00225 M).
Answer for screen readers
To compare the precision of the indicators, compare their standard deviations. At a 95% confidence level:
- 1 vs 2: Indicator 2 (Methyl red, SD = 0.00098 M) is more precise than Indicator 1 (Bromothymol blue, SD = 0.00225 M).
- 2 vs 3: Indicator 2 (Methyl red, SD = 0.00098 M) is more precise than Indicator 3 (Bromocresol green, SD = 0.00113 M).
- 1 vs 3: Indicator 3 (Bromocresol green, SD = 0.00113 M) is more precise than Indicator 1 (Bromothymol blue, SD = 0.00225 M).
More Information
Precision reflects how close multiple measurements are to each other. Standard deviation (SD) quantifies this, with a smaller SD indicating greater precision. A confidence level of 95% is a common statistical measure.
Tips
A common mistake is to confuse precision with accuracy. Precision refers to the repeatability of a measurement, while accuracy refers to how close the measurement is to the true value.
Sources
AI-generated content may contain errors. Please verify critical information