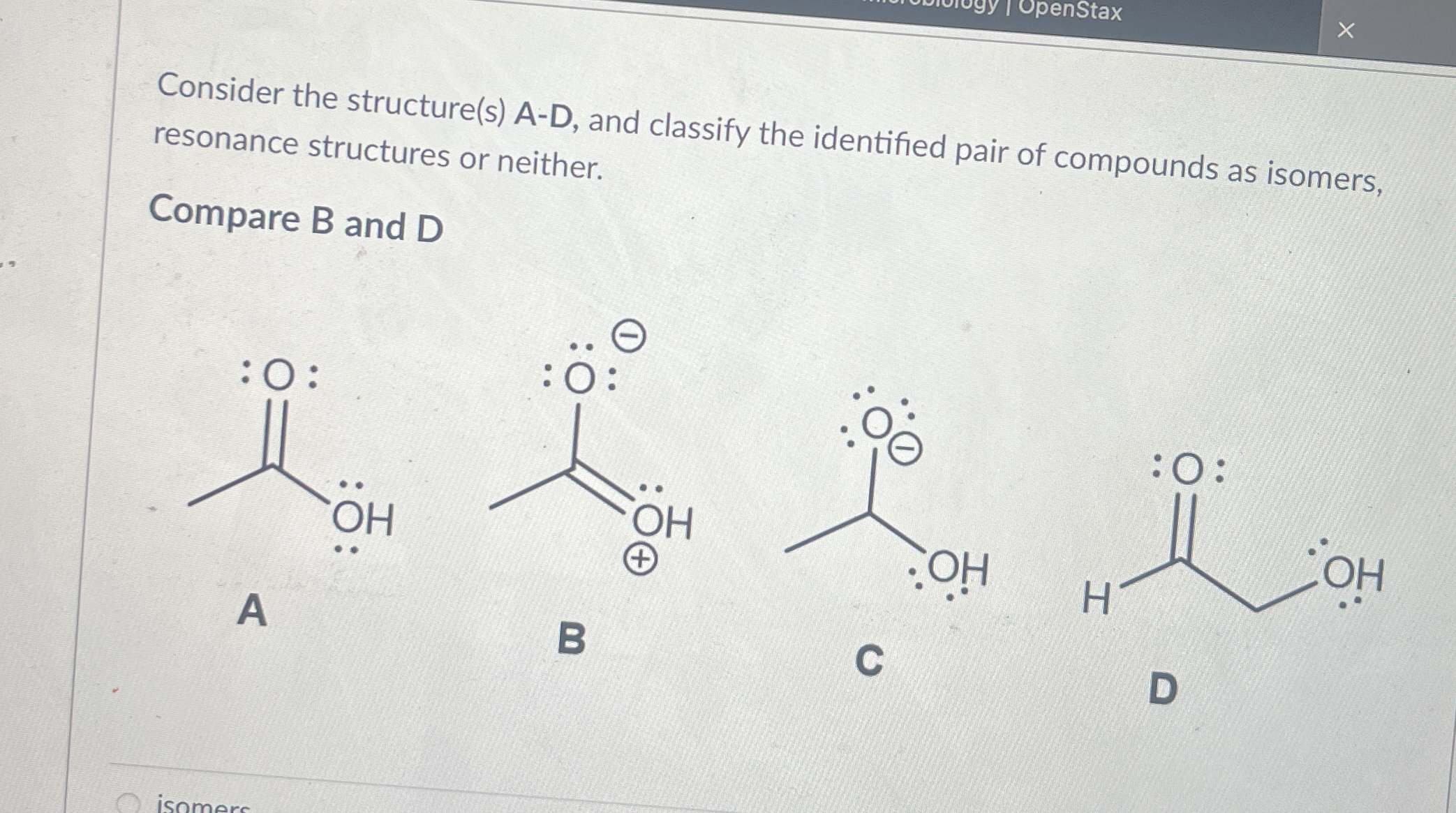
Compare B and D and classify the identified pair of compounds as isomers, resonance structures or neither.
Understand the Problem
The question is asking to compare two chemical structures, B and D, to determine if they are isomers, resonance structures, or neither. This involves understanding chemical structures and their properties.
Answer
B and D are isomers.
The final answer is that B and D are isomers.
Answer for screen readers
The final answer is that B and D are isomers.
More Information
Isomers are compounds with the same molecular formula but different arrangements of atoms. This is in contrast to resonance structures, which involve the same arrangement of atoms but different electron positions.
Tips
A common mistake is confusing resonance structures with isomers. Remember that resonance structures only differ in electron placement, while isomers differ in atom arrangement.
Sources
AI-generated content may contain errors. Please verify critical information