Can you provide notes on giant structures in ionic and covalent compounds for my test tomorrow?
Understand the Problem
The question is asking for notes or summaries related to giant structures in ionic and covalent compounds, specifically focusing on sodium chloride and carbon structures like diamond. The information likely pertains to a science test.
Answer
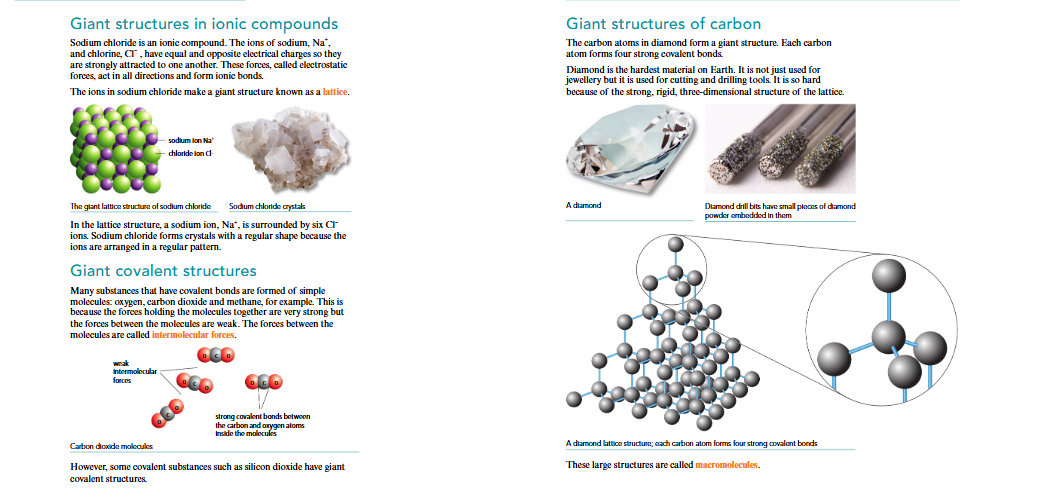
Giant ionic structures form lattices with strong electrostatic forces, while giant covalent structures involve continuous networks of covalent bonds.
Giant ionic structures like sodium chloride are lattices with strong electrostatic forces between ions. Giant covalent structures, such as diamond and silicon dioxide, have strong covalent bonds forming a continuous network. They can withstand high temperatures and pressures without breaking.
Answer for screen readers
Giant ionic structures like sodium chloride are lattices with strong electrostatic forces between ions. Giant covalent structures, such as diamond and silicon dioxide, have strong covalent bonds forming a continuous network. They can withstand high temperatures and pressures without breaking.
More Information
Giant ionic structures, like sodium chloride, are highly stable due to their strong ionic bonds. Giant covalent structures include diamond and silicon dioxide, known for their hardness and high melting points due to the strong covalent bonds.
Tips
A common mistake is confusing the lattice structure in ionic compounds with molecular structures. Ensure you understand the distinction between ionic lattices and covalent networks.
Sources
- Giant covalent structures - BBC Bitesize - bbc.co.uk
- Properties of Giant Covalent Structures (GCSE Chemistry) - studymind.co.uk
- Comparing Ionic & Covalent Compounds | Edexcel GCSE Chemistry - savemyexams.com
AI-generated content may contain errors. Please verify critical information