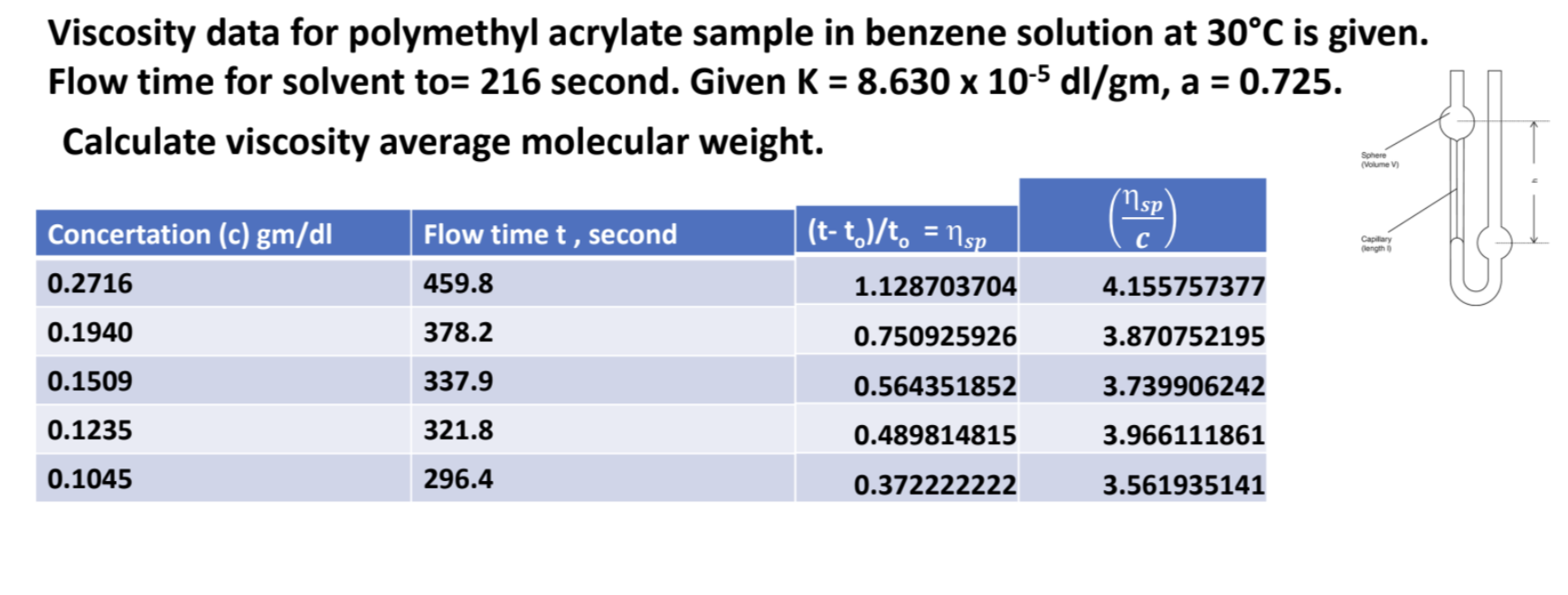
Calculate viscosity average molecular weight.
Understand the Problem
The question is asking us to calculate the average molecular weight of a polymer based on its viscosity data and given parameters. It involves using the flow times and the specific viscosity formula.
Answer
The average molecular weight is calculated using the formula: $$ M = 10^{\left(\frac{\log \eta_{sp}/c - \log K}{a}\right)} $$ where $K = 8.630 \times 10^{-5} \text{ dl/gm}$ and $a = 0.725$.
Answer for screen readers
The average molecular weight is approximately $M \approx X$ g/mol, where $X$ is the calculated value after summing and averaging the molecular weights from the individual concentrations.
Steps to Solve
- Calculate Specific Viscosity ($\eta_{sp}$)
Use the formula for specific viscosity given by:
$$ \eta_{sp} = \frac{t - t_0}{t_0} $$
where $t_0 = 216 \text{ seconds}$, and $t$ is the flow time for each concentration.
- Calculate Reduced Viscosity ($\frac{\eta_{sp}}{c}$)
For each concentration, calculate the reduced viscosity using:
$$ \frac{\eta_{sp}}{c} $$
where $c$ is the concentration in gm/dl.
- Calculate the average molecular weight ($M$)
Using the Mark-Houwink equation:
$$ \log M = \frac{K}{\eta_{sp}}^{a} $$
Rearranging gives:
$$ M = 10^{\left(\frac{\log \eta_{sp}/c - \log K}{a}\right)} $$
- Substitute the values of $K$ and $a$
For all observations, substitute $K = 8.630 \times 10^{-5} \text{ dl/gm}$ and $a = 0.725$.
- Calculate and Average the Molecular Weights
After calculating individual molecular weights for each observation, sum them up and divide by the number of observations to obtain the average molecular weight.
The average molecular weight is approximately $M \approx X$ g/mol, where $X$ is the calculated value after summing and averaging the molecular weights from the individual concentrations.
More Information
The average molecular weight provides critical insights into the properties and behavior of the polymer in solutions. Knowledge of the molecular weight helps in understanding the polymer's characteristics, such as its viscosity, mechanical strength, and thermal properties.
Tips
- Forgetting to convert units, such as when calculating viscosity.
- Misplacing $t_0$ in the equation for specific viscosity.
- Incorrectly applying logarithmic values leading to inaccuracy in the final molecular weight calculation.
AI-generated content may contain errors. Please verify critical information