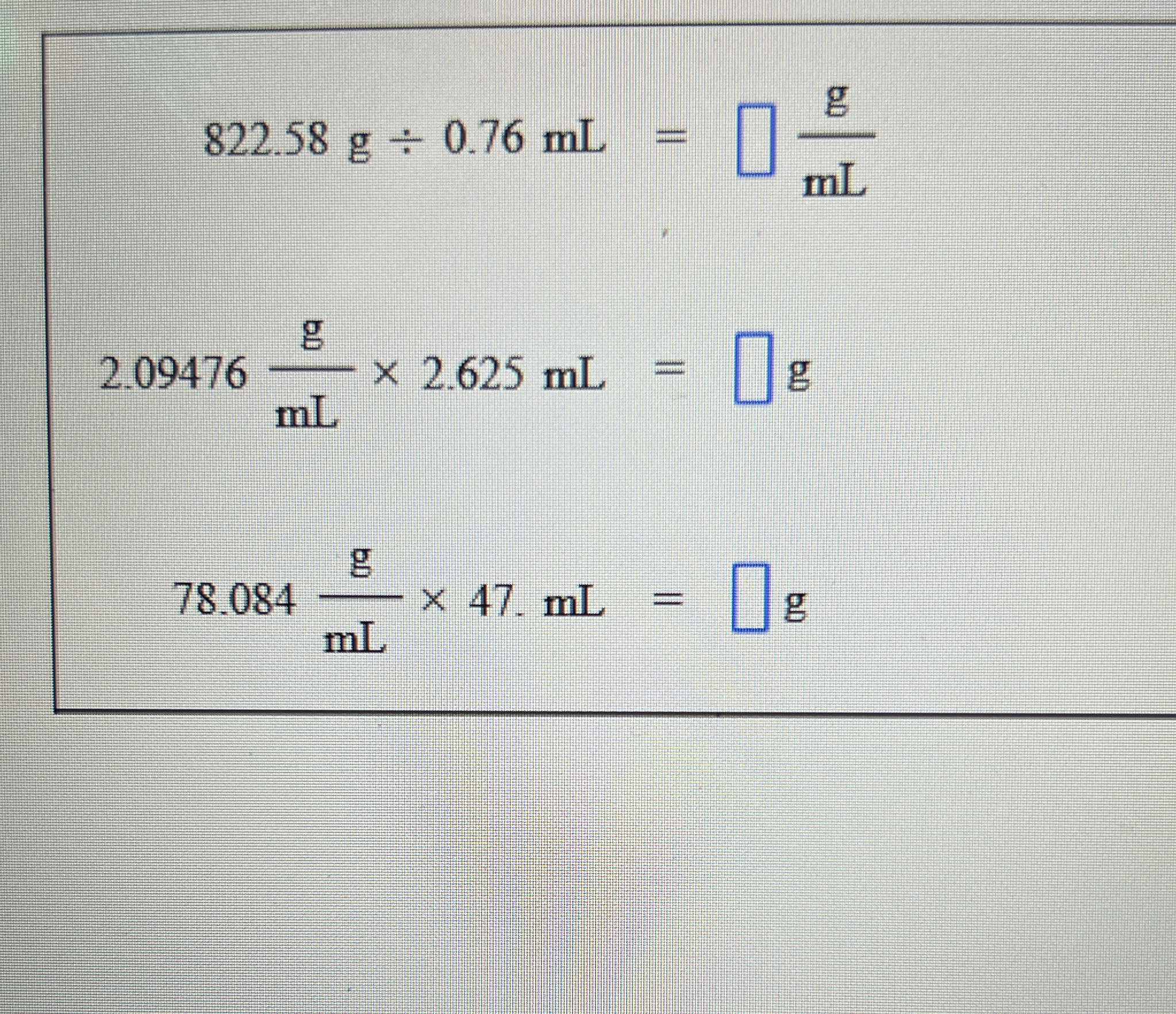
Calculate the results for the following expressions: 822.58 g ÷ 0.76 mL, 2.09476 g/mL × 2.625 mL, 78.084 g/mL × 47 mL.
Understand the Problem
The question presents multiple calculations involving mass, volume, and density. It requires solving for missing values using the given formulas, which appear to involve density in terms of grams per milliliter (g/mL).
Answer
1. $1082.13 \, \text{g/mL}$ 2. $5.50 \, \text{g}$ 3. $3667.95 \, \text{g}$
Answer for screen readers
-
The density is approximately $1082.13 , \text{g/mL}$.
-
The mass is approximately $5.50 , \text{g}$.
-
The mass is approximately $3667.95 , \text{g}$.
Steps to Solve
- Calculate Density
To find the density in g/mL, divide the mass by the volume:
$$ \text{Density} = \frac{822.58 , \text{g}}{0.76 , \text{mL}} $$
- Calculate Mass
To find the mass, multiply the density by the volume:
$$ \text{Mass} = 2.09476 , \frac{\text{g}}{\text{mL}} \times 2.625 , \text{mL} $$
- Calculate Mass Again
For the third calculation, again multiply density by volume:
$$ \text{Mass} = 78.084 , \frac{\text{g}}{\text{mL}} \times 47 , \text{mL} $$
-
The density is approximately $1082.13 , \text{g/mL}$.
-
The mass is approximately $5.50 , \text{g}$.
-
The mass is approximately $3667.95 , \text{g}$.
More Information
- The calculations rely on the relationship between mass, volume, and density.
- Density is typically used in chemistry to understand how much mass is contained in a given volume of a substance.
Tips
- Not converting units correctly, especially if the volume is not in mL or mass is not in grams.
- Miscalculating by reversing the mass and volume relationships.
AI-generated content may contain errors. Please verify critical information