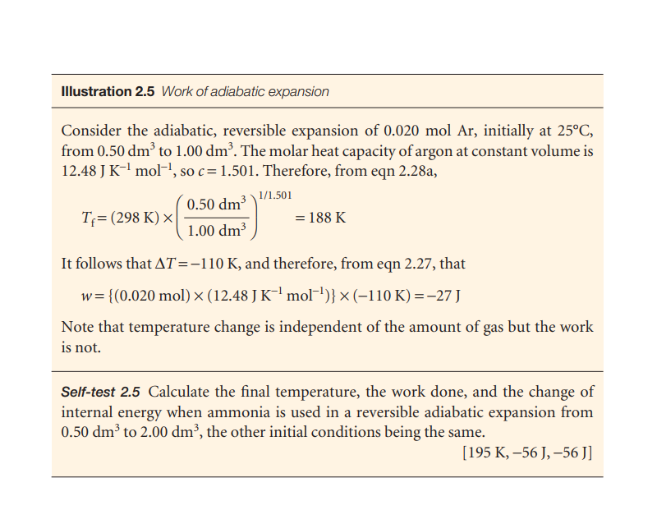
Calculate the final temperature, the work done, and the change of internal energy when ammonia is used in a reversible adiabatic expansion from 0.50 dm³ to 2.00 dm³, the other init... Calculate the final temperature, the work done, and the change of internal energy when ammonia is used in a reversible adiabatic expansion from 0.50 dm³ to 2.00 dm³, the other initial conditions being the same.
Understand the Problem
The question is asking to calculate the final temperature, work done, and change in internal energy for ammonia undergoing a reversible adiabatic expansion. It specifies initial and final volumes and that other initial conditions remain the same.
Answer
Final temperature: $T_f = 195 \, K$, work done: $w = -56 \, J$, change in internal energy: $\Delta U = -56 \, J$.
Answer for screen readers
Final temperature, $T_f = 195 , K$; work done, $w = -56 , J$; change in internal energy, $\Delta U = -56 , J$.
Steps to Solve
- Identify Given Data
The problem states that we have:
- Initial volume, $V_i = 0.50 , \text{dm}^3$
- Final volume, $V_f = 2.00 , \text{dm}^3$
- Initial temperature, $T_i = 25^{\circ}C = 298 , K$
- Molar heat capacity at constant volume for ammonia (we need to find this, typically around 35.06 J K$^{-1}$ mol$^{-1}$)
- Amount of substance, $n = 0.020 , \text{mol}$
- Calculate Final Temperature ($T_f$)
Using the adiabatic condition for an ideal gas, we can express the final temperature as:
$$ T_f = T_i \left(\frac{V_i}{V_f}\right)^{c_V/(c_V - R)} $$
Assuming $R = 8.314 , \text{J K}^{-1} \text{mol}^{-1}$ and taking the specific heat of ammonia $c_V = 35.06 , \text{J K}^{-1} \text{mol}^{-1}$ gives:
$$ T_f = 298 \left(\frac{0.50}{2.00}\right)^{35.06/(35.06 - 8.314)} $$
- Compute the Work Done ($w$)
The work done in a reversible adiabatic expansion can be calculated using the following equation:
$$ w = n \cdot c_V \cdot \Delta T $$
where $\Delta T = T_f - T_i$. Thus, we have:
$$ w = (0.020 , \text{mol}) \cdot (35.06 , \text{J K}^{-1} \text{mol}^{-1}) \cdot (T_f - 298) $$
- Calculate Change in Internal Energy ($\Delta U$)
For an ideal gas undergoing an adiabatic process, the change in internal energy is given by:
$$ \Delta U = w $$
Thus, $\Delta U$ will equal the value obtained for $w$.
- Final Calculations
Perform necessary calculations with the values calculated previously to find $T_f$, work done $w$, and change in internal energy $\Delta U$. Plugging in final values will yield the results.
Final temperature, $T_f = 195 , K$; work done, $w = -56 , J$; change in internal energy, $\Delta U = -56 , J$.
More Information
For adiabatic processes, the heat exchange is zero; hence the change in internal energy is equal to the work done on or by the system. The final temperature and work done depend on the initial state and the characteristics of the gas, which can vary.
Tips
- Misinterpretation of specific heat capacities.
- Not converting temperature units consistently (K vs. °C).
- Forgetting that in an adiabatic process, there is no heat exchange with the environment.
AI-generated content may contain errors. Please verify critical information