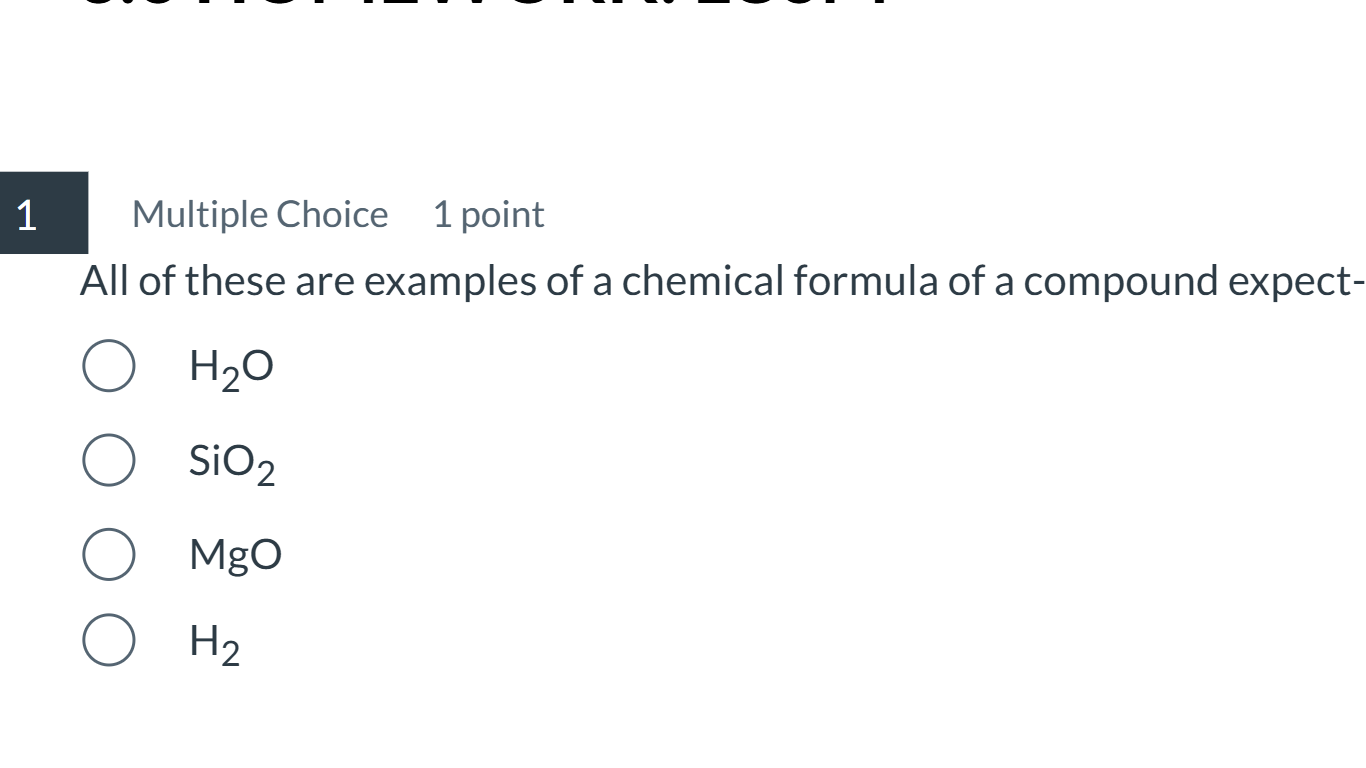
All of these are examples of a chemical formula of a compound except: H2O SiO2 MgO H2
Understand the Problem
The question asks us to identify which of the given chemical formulas is NOT a compound, given H2O, SiO2, MgO and H2.
Answer
H2
The correct answer is H2. A chemical compound is composed of two or more different elements. H2 is an element.
Answer for screen readers
The correct answer is H2. A chemical compound is composed of two or more different elements. H2 is an element.
More Information
A compound requires two elements, while H2 is only hydrogen.
Tips
A common mistake is to assume any combination of atoms is a compound; however, it must be different elements.
Sources
- Chemical compound | Definition, Examples, & Types - Britannica - britannica.com
AI-generated content may contain errors. Please verify critical information