2.09476 g/mL × 2.625 mL = ? g
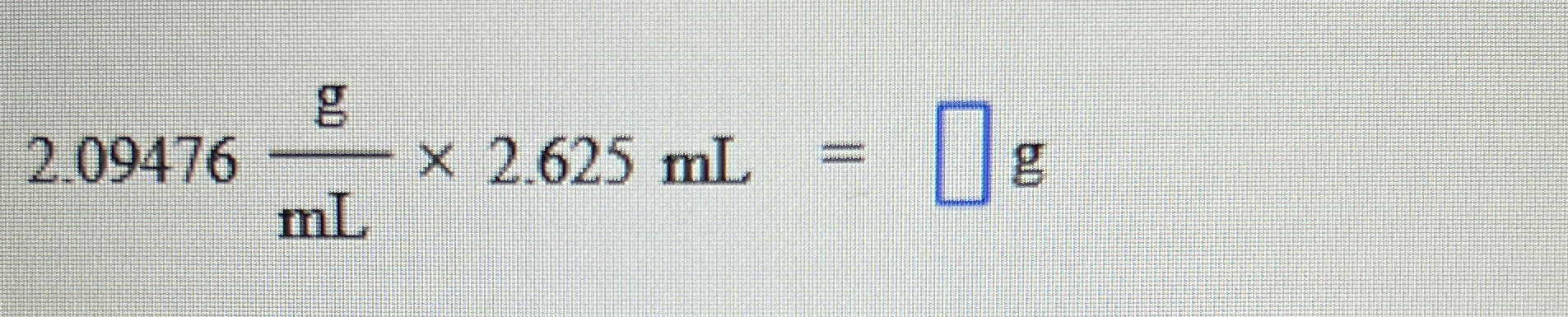
Understand the Problem
The question involves performing a calculation to find the mass in grams equivalent to a volume of 2.625 mL, given a density of 2.09476 g/mL. We will use the formula: mass = density × volume.
Answer
The mass is approximately $5.50 \, \text{g}$.
Answer for screen readers
The mass is approximately $5.50 , \text{g}$.
Steps to Solve
-
Identify the Formula To find the mass, use the formula: $$ \text{mass} = \text{density} \times \text{volume} $$
-
Plug in the Values Substitute the values for density and volume into the formula: $$ \text{mass} = 2.09476 , \text{g/mL} \times 2.625 , \text{mL} $$
-
Perform the Multiplication Calculate the mass: $$ \text{mass} = 2.09476 \times 2.625 $$
-
Calculate the Result Using a calculator or doing the multiplication manually: $$ \text{mass} = 5.496493 $$
-
Round the Answer Round the answer to an appropriate number of significant figures, usually matching the least precise measurement: $$ \text{mass} \approx 5.50 , \text{g} $$
The mass is approximately $5.50 , \text{g}$.
More Information
This calculation is a straightforward application of the formula for density. Knowing the density of a substance allows for the determination of mass if the volume is known.
Tips
- Forgetting to multiply correctly; make sure to do the arithmetic precisely.
- Not accounting for significant figures in the final answer; always match the precision of your result to the least precise measurement.
AI-generated content may contain errors. Please verify critical information