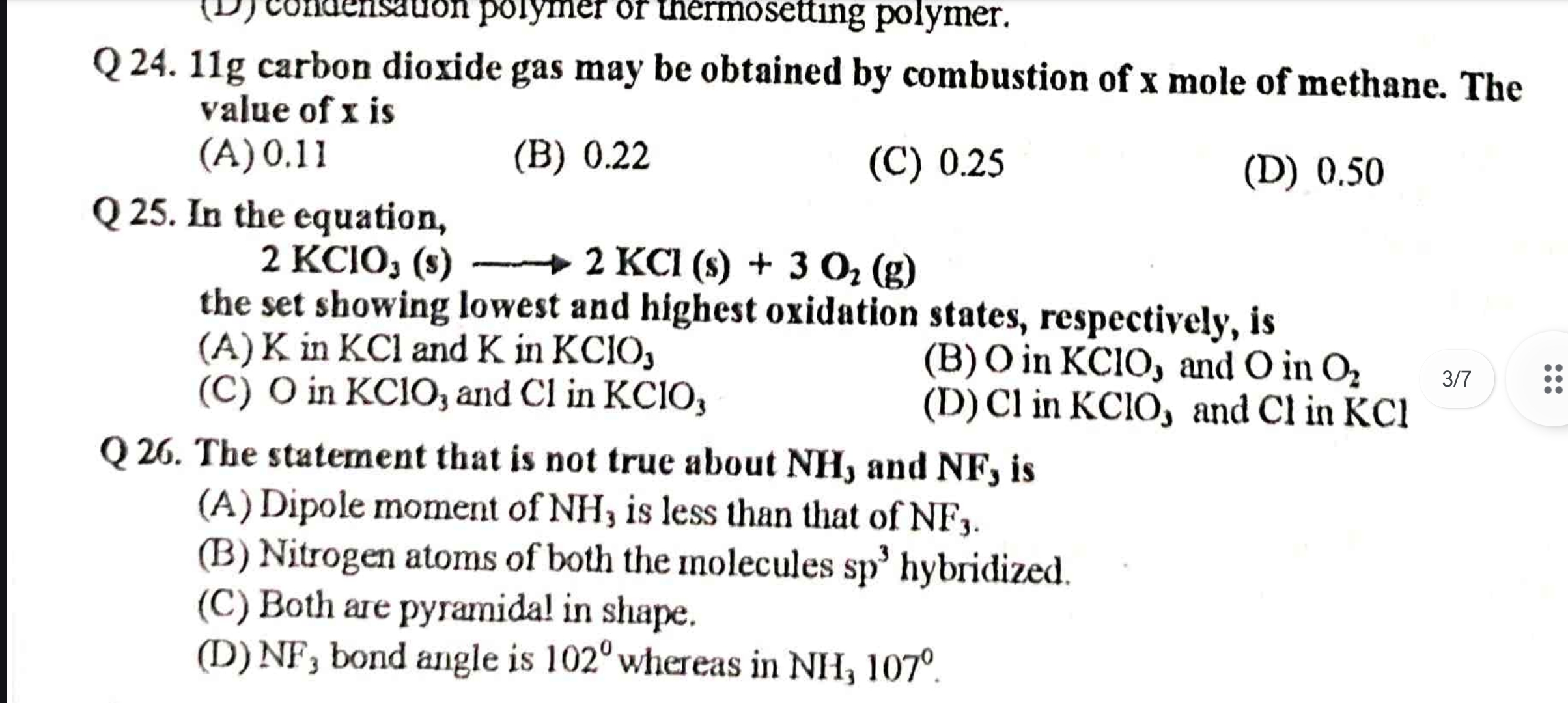
11g carbon dioxide gas may be obtained by combustion of x mole of methane. The value of x is. In the equation, 2 KClO3 (s) ⇌ 2 KCl (s) + 3 O2 (g) the set showing lowest and highest... 11g carbon dioxide gas may be obtained by combustion of x mole of methane. The value of x is. In the equation, 2 KClO3 (s) ⇌ 2 KCl (s) + 3 O2 (g) the set showing lowest and highest oxidation states, respectively, is. The statement that is not true about NH3 and NF3 is.
Understand the Problem
The question presents multiple choice options regarding the combustion of methane to produce carbon dioxide, the oxidation states of elements in a chemical reaction, and comparisons between the molecular geometries and bond angles of NH3 and NF3. It aims to test knowledge in chemistry, specifically in the areas of gas laws, chemical reactions, and molecular structure.
Answer
x = 0.25, Oxidation states: D, False about NH3 and NF3: A
The value of x is 0.25. The set showing the lowest and highest oxidation states is Cl in KCl and Cl in KClO3. The incorrect statement is that the dipole moment of NH3 is less than NF3.
Answer for screen readers
The value of x is 0.25. The set showing the lowest and highest oxidation states is Cl in KCl and Cl in KClO3. The incorrect statement is that the dipole moment of NH3 is less than NF3.
More Information
1 mole CH4 produces 1 mole CO2; 11 g CO2 is 0.25 moles. KClO3 oxidation changes from +1 to +5. NH3 has a higher dipole moment due to higher electronegativity difference.
Tips
Common mistake is forgetting the 1:1 molar ratio in CH4 and CO2 or miscalculating oxidation states.
Sources
- If 11 grams of carbon dioxide is produced, how much methane, "CH ... - socratic.org
- Oxidation States of Transition Metals - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information