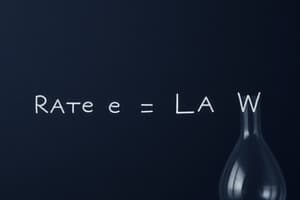Podcast
Questions and Answers
What does the rate law or rate equation for a chemical reaction link the rate of forward reaction with?
What does the rate law or rate equation for a chemical reaction link the rate of forward reaction with?
- The volumes of the reactants
- The masses of the reactants
- The concentrations or pressures of the reactants and constant parameters (correct)
- The temperatures of the reactants
What does the expression $v_{0};=;k[\mathrm {A} ]^{x}[\mathrm {B} ]^{y}$ represent?
What does the expression $v_{0};=;k[\mathrm {A} ]^{x}[\mathrm {B} ]^{y}$ represent?
- The equilibrium constant of a chemical reaction
- The initial rate of a chemical reaction (correct)
- The final rate of a chemical reaction
- The rate of reverse reaction
What do the exponents $x$ and $y$ in the rate equation represent?
What do the exponents $x$ and $y$ in the rate equation represent?
- The concentrations of the reactants A and B
- The rate coefficients for A and B
- The pressures of the reactants A and B
- The partial orders of reaction for A and B (correct)
What is the overall reaction order in the rate equation?
What is the overall reaction order in the rate equation?
What can the values of the exponents in the rate equation be?
What can the values of the exponents in the rate equation be?
Flashcards are hidden until you start studying