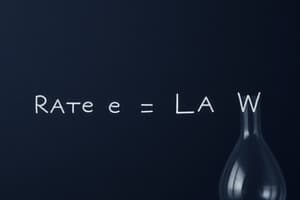Podcast
Questions and Answers
Why are common names generally unsuitable for scientific communication when identifying new species?
Why are common names generally unsuitable for scientific communication when identifying new species?
- They can be ambiguous and vary by region or language. (correct)
- They provide detailed information about the species' evolutionary history.
- They are universally recognized across different languages.
- They are regulated by international scientific organizations.
What is the correct format for writing a scientific name using binomial nomenclature?
What is the correct format for writing a scientific name using binomial nomenclature?
- _Genus species_ (Genus capitalized, species lowercase and both italicized) (correct)
- genus species (both lowercase)
- _Genus species_ (Genus capitalized and italicized)
- Genus species (both capitalized)
Which two levels of taxa are considered the broadest (highest) in the Linnaean system of classification?
Which two levels of taxa are considered the broadest (highest) in the Linnaean system of classification?
- Domain and Kingdom (correct)
- Class and Order
- Kingdom and Phylum
- Family and Genus
Traditional classifications considered which two primary aspects when grouping organisms?
Traditional classifications considered which two primary aspects when grouping organisms?
In Linnaeus' original system, into which two kingdoms was all life divided?
In Linnaeus' original system, into which two kingdoms was all life divided?
Organisms in the kingdoms Eubacteria and Archaebacteria are now grouped into what higher-level taxonomic classification?
Organisms in the kingdoms Eubacteria and Archaebacteria are now grouped into what higher-level taxonomic classification?
What is the primary characteristic of organisms that belong to the same species?
What is the primary characteristic of organisms that belong to the same species?
Why is the use of common names problematic in scientific research?
Why is the use of common names problematic in scientific research?
Which of the following best describes the relationship between taxa levels in the Linnaean system?
Which of the following best describes the relationship between taxa levels in the Linnaean system?
How does the concept of 'common ancestry' relate to the construction of cladograms?
How does the concept of 'common ancestry' relate to the construction of cladograms?
Flashcards
Why avoid common names?
Why avoid common names?
Common names are not useful for scientists due to potential for confusion and ambiguity.
Linnaean system of classification
Linnaean system of classification
The Linnaean system involves a hierarchical classification of organisms into nested groups based on shared characteristics.
Binomial Nomenclature
Binomial Nomenclature
Binomial nomenclature is the two-word naming format where species are named; first the genus, then the specific epithet.
Highest Linnaean Taxa
Highest Linnaean Taxa
Signup and view all the flashcards
Traditional Classification Factors
Traditional Classification Factors
Signup and view all the flashcards
Linnaeus' Kingdoms
Linnaeus' Kingdoms
Signup and view all the flashcards
Previous Grouping of Bacteria
Previous Grouping of Bacteria
Signup and view all the flashcards
Unicellular Domains
Unicellular Domains
Signup and view all the flashcards
Genus vs Species
Genus vs Species
Signup and view all the flashcards
Derived Character
Derived Character
Signup and view all the flashcards
Study Notes
Chemical Kinetics
- Reaction rate defined as the change in concentration of reactants or products per unit time.
Rate Law
- The rate law ($rate = k[A]^m[B]^n$) expresses the relationship between the rate of a reaction and the concentrations of the reactants.
- $k$ is the rate constant.
- [A] and [B] are concentrations of reactants.
- $m$ and $n$ are reaction orders with respect to A and B.
Reaction Order
- Reaction order is the sum of the exponents in the rate law (m + n).
- Zero order: rate = k (rate is independent of reactant concentration).
- First order: rate = k[A] (rate is directly proportional to the concentration of A).
- Second order: rate = k[A]^2 or rate = k[A][B].
Factors Affecting Reaction Rate
Temperature
-
Temperature affects reaction rate
-
Arrhenius Equation: $k = Ae^{-E_a/RT}$
- $k$ is the rate constant
- $A$ is the pre-exponential factor
- $E_a$ is the activation energy
- $R$ is the gas constant ($8.314 J/(mol \cdot K)$)
- $T$ is the temperature in Kelvin
Catalysts
- Catalysts lower the activation energy ($E_a$) of a reaction, and increasing the reaction rate.
Reaction Mechanisms
Elementary Steps
- Elementary step constitutes a single step in a reaction mechanism
Rate-Determining Step
- Rate-determining step involves the slowest step in a reaction mechanism which determine the overall rate of the reaction.
Equilibrium
Equilibrium Constant
- For a reversible reaction $aA + bB \rightleftharpoons cC + dD$, the equilibrium constant ($K_c$) is $K_c = \frac{{[C]^c[D]^d}}{{[A]^a[B]^b}}$.
Le Chatelier's Principle
- Le Chatelier's Principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress.
Changes in Condition
- Concentration: Adding reactants will shift the equilibrium towards products, and vice versa.
- Pressure: Increasing pressure will shift the equilibrium towards the side with fewer moles of gas, and vice versa.
- Temperature: Increasing temperature will favor the endothermic reaction, and vice versa.
Acid-Base Chemistry
Definitions
- Arrhenius Acid: Produces $H^+$ ions in water.
- Arrhenius Base: Produces $OH^-$ ions in water.
- Bronsted-Lowry Acid: Donates a proton ($H^+$).
- Bronsted-Lowry Base: Accepts a proton ($H^+$).
- Lewis Acid: Accepts an electron pair.
- Lewis Base: Donates an electron pair.
pH Scale
- $pH = -log[H^+]$
- $pOH = -log[OH^-]$
- $pH + pOH = 14$ at $25^\circ C$
Acid-Base Strength
- Strong Acids: Completely dissociate in water (e.g., $HCl, H_2SO_4$)
- Strong Bases: Completely dissociate in water (e.g., $NaOH, KOH$)
- Weak Acids/Bases: Partially dissociate in water.
Acid Dissociation Constant ($K_a$)
- For a weak acid $HA$, $K_a = \frac{{[H^+][A^-]}}{{[HA]}}$ and $pK_a = -log(K_a)$.
Base Dissociation Constant ($K_b$)
- For a weak base B, $K_b = \frac{{[BH^+][OH^-]}}{{[B]}}$ and $pK_b = -log(K_b)$.
Relationship between $K_a$ and $K_b$
- For a conjugate acid-base pair, $K_a \cdot K_b = K_w$, where $K_w$ is the ion product of water ($1.0 \times 10^{-14}$ at $25^\circ C$)
Buffers
- A buffer is a solution that resists changes in pH when small amounts of acid or base are added.
Henderson-Hasselbalch Equation
- For acidic buffer: $pH = pK_a + log \frac{{[A^-]}}{{[HA]}}$
- For basic buffer: $pOH = pK_b + log \frac{{[BH^+]}}{{[B]}}$
Titration
- Titration involves a process used to determine the concentration of a solution by reacting it with a solution of known concentration.
Equivalence Point
- Equivalence point denotes the point in a titration where the acid and base have completely reacted.
Endpoint
- Endpoint is the point in a titration where the indicator changes color.
Indicators
- Indicators are substances that change color depending on the pH of the solution.
Biochemistry I
Amino Acids
- Amino acids are the building blocks of proteins and proteins being involved in almost every function of the human body.
- There are 20 common amino acids.
General Formula
- The general formula of an amino acid is $NH_2-CHR-COOH$, with each amino acid has a different "R" group.
Classes of Amino Acids
Nonpolar, Aliphatic R Groups
- These are hydrophobic, including Glycine, Alanine, Proline, Valine, Leucine, Isoleucine, Methionine.
Aromatic R Groups
- These are relatively nonpolar, which include Phenylalanine, Tyrosine, Tryptophan.
Polar, Uncharged R Groups
- These are hydrophilic, including Serine, Threonine, Cysteine, Asparagine, Glutamine.
Positively Charged R Groups
- These are hydrophilic and involves Lysine, Arginine, Histidine.
Negatively Charged R Groups
- These are hydrophilic, involving Aspartate, Glutamate.
Titration of Amino Acids
- Amino acids are weak acids having at least two dissociable protons, $H^+$.
- Titration curve reveals two distinct stages of deprotonation when an amino acid is titrated with a base, such as $NaOH$.
Peptide Bond Formation
- Amino acids can be linked together through peptide bonds to form peptides and proteins.
- A peptide bond is an amide bond between the $\alpha$-carboxyl group of one amino acid and the $\alpha$-amino group of another.
- The formation of a peptide bond involves the loss of a molecule of water.
Protein Structure
Primary Structure
- It represents the linear sequence of amino acids.
- It is determined by by the sequence of the amino acids in the polypeptide chain.
- It determines the higher levels of protein structure.
Secondary Structure
- Secondary structure: local spatial arrangement of the polypeptide backbone.
- The two most common types of secondary structure: $\alpha$-helix and $\beta$-sheet.
Tertiary Structure
- Tertiary Structure involves the overall three-dimensional arrangement of all the atoms in the protein.
- Variety of interactions stabilizees the tertiary structure, involving hydrophobic interactions, hydrogen bonds, disulfide bonds, and ionic interactions.
Quaternary Structure
- Quaternary structure represents the arrangement of multiple polypeptide chains in a multi-subunit protein.
- Proteins with quaternary structure have two or more polypeptide chains, called subunits, which are held together by the same types of interactions that stabilize tertiary structure.
Protein Folding
- Protein folding involves the process in which a protein acquires its native three-dimensional structure.
- This is driven by the hydrophobic effect, where nonpolar amino acids will cluster in the interior of the protein.
- Protein folding is often assisted by chaperone proteins, which prevent misfolding and aggregation.
Protein Misfolding and Disease
- Protein misfolding can lead to a variety of diseases, including Alzheimer's disease, Parkinson's disease, and Huntington's disease.
- In these diseases, misfolded proteins aggregate to form amyloid plaques.
Enzymes
- Enzymes biological catalysts that speed up the rate of chemical reactions in living organisms.
- Enzymes made of proteins.
Enzyme Kinetics
-
Enzyme kinetics involves the study of the rates of enzyme-catalyzed reactions.
-
Michaelis-Menten equation constitutes a mathematical model that describes the kinetics of many enzymes, where $V = \frac{V_{max}[S]}{K_m + [S]}$
- $V$ is the initial reaction rate
- $V_{max}$ is the maximum reaction rate
- $[S]$ is the substrate concentration
- $K_m$ is the Michaelis constant
Enzyme Inhibition
- Enzyme inhibitors are molecules that reduce the activity of enzymes and can be competitive, uncompetitive, or noncompetitive. Enzymes can be inhibited by molecules.
Regulation of Enzyme Activity
- Enzyme activity can be regulated by a variety of mechanisms, including:
- Allosteric control
- Covalent modification
- Proteolytic cleavage
- Changes in enzyme concentration
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.