Podcast
Questions and Answers
Molecules that have the same chemical formula but different three-dimensional shapes are called ____.
Molecules that have the same chemical formula but different three-dimensional shapes are called ____.
- Enantiomers
- Functional groups
- Isomers (correct)
- Isotopes
- Hydrocarbons
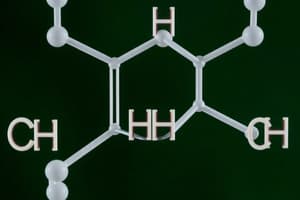
Which of the following best describes cis-trans isomers?
Which of the following best describes cis-trans isomers?
- They differ in their spatial arrangement around inflexible double bonds. (correct)
- They are long chains of hydrogen and carbon atoms.
- They are mirror images of each other.
- They differ in the arrangement of covalent bonds and in covalent partners.
- They have the same number of atoms of the same elements but different structures.
What is an amino group?
What is an amino group?
NH2
What is a phosphate group?
What is a phosphate group?
What is a hydroxyl group?
What is a hydroxyl group?
What is a carboxyl group?
What is a carboxyl group?
What functional group is characteristic of alcohol?
What functional group is characteristic of alcohol?
Which functional group tends to behave as a base?
Which functional group tends to behave as a base?
What characterizes thiols?
What characterizes thiols?
What characterizes phosphate groups?
What characterizes phosphate groups?
What is a carbonyl group?
What is a carbonyl group?
Which of the following functional groups behaves as an acid?
Which of the following functional groups behaves as an acid?
Variations in the reactive properties of different organic molecules are most closely associated with ____.
Variations in the reactive properties of different organic molecules are most closely associated with ____.
Which polymers are composed of amino acids?
Which polymers are composed of amino acids?
Enzymes in the digestive tract catalyze hydrolysis reactions.
Enzymes in the digestive tract catalyze hydrolysis reactions.
Which of the following statements about the formation of polypeptides from amino acids is true?
Which of the following statements about the formation of polypeptides from amino acids is true?
What level of protein structure is characterized by the linear sequence of amino acids?
What level of protein structure is characterized by the linear sequence of amino acids?
What is a dehydration reaction?
What is a dehydration reaction?
Which representation of protein structure is most useful for observing the path of the protein chain?
Which representation of protein structure is most useful for observing the path of the protein chain?
Which representation of protein structure is most useful for studying the exterior of the protein?
Which representation of protein structure is most useful for studying the exterior of the protein?
Flashcards are hidden until you start studying
Study Notes
Isomers and Functional Groups
- Isomers are molecules with the same chemical formula but different structures, resulting in varying properties.
- Cis-trans isomers are a type of isomer that differ in spatial arrangement around inflexible double bonds.
- Functional groups include various specific atom arrangements that influence molecular properties and reactions.
- An amino group is identified by the presence of NH2, while a phosphate group contains phosphorus and is central to energy transfer in ATP.
- Hydroxyl groups (–OH) characterize alcohols, while carboxyl groups (–COOH) can act as acids by donating hydrogen ions.
Polymers and Monomers
- Polymers are large molecules formed by bonding smaller units known as monomers.
- Proteins are unique polymers composed of amino acid monomers linked together through peptide bonds.
- Amino acids always contain an amino functional group, a carboxyl functional group, a side chain (“R group”), and hydrogen.
Amino Acids and Protein Structure
- The carboxyl functional group confers acidity to an amino acid, while a methyl group consists of a carbon bonded to three hydrogens.
- RNA is formed from nucleotide monomers, whereas proteins consist of amino acids.
- Protein structures are classified into four levels: primary (amino acid sequence), secondary (alpha-helices and beta-sheets), tertiary (3D folding due to interactions among R groups), and quaternary (assembly of multiple polypeptide chains).
- A dehydration reaction occurs when amino acids bond, forming a peptide bond while releasing water.
Protein Visualization and Function
- Protein structures can be represented in different ways to highlight specific aspects: backbone models trace the chain, ribbon models show folds, and space-fill models display the exterior shape.
- Enzymatic proteins, like lysozyme, catalyze chemical reactions and are crucial for processes such as digestion.
- The characteristics of side chains in proteins determine their behavior in different environments, such as hydrophobic or hydrophilic interactions.
Summary of Protein Structure
- Primary structure refers to the specific sequence of amino acids. Secondary structure arises from hydrogen bonds between nearby backbone atoms. Tertiary structure results from R group interactions, while quaternary structure involves multiple subunits.
- The unique arrangement of amino acids in proteins drives their functional diversity, despite sharing the same number of each type of amino acid across different proteins.
Key Concepts in Biochemistry
- Enzymes increase reaction rates through catalysis and are not consumed in the process.
- The diverse properties of organic molecules are linked to the presence and arrangement of functional groups.
- The nature of R groups in amino acids defines their classification as neutral, acidic, or basic, influencing protein interactions and functionality within cells.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.