Podcast
Questions and Answers
What is the primary function of the pedicles in a vertebra?
What is the primary function of the pedicles in a vertebra?
- To protect the spinal cord and nerves
- To connect the vertebra to the posterior elements (correct)
- To serve as attachment points for muscles and ligaments
- To support the body and maintain posture
Which region of the spine has the largest vertebrae?
Which region of the spine has the largest vertebrae?
- Sacrum
- Thoracic
- Lumbar (correct)
- Cervical
What is the purpose of the transverse processes in a vertebra?
What is the purpose of the transverse processes in a vertebra?
- To support the body and maintain posture
- To protect the spinal cord and nerves
- To serve as attachment points for muscles and ligaments (correct)
- To connect the vertebra to the posterior elements
How many vertebrae are in the sacrum?
How many vertebrae are in the sacrum?
What is the main part of the vertebra that bears weight?
What is the main part of the vertebra that bears weight?
Which region of the spine has the most limited motion?
Which region of the spine has the most limited motion?
What is the function of the spinous process in a vertebra?
What is the function of the spinous process in a vertebra?
How many vertebrae are in the human spine?
How many vertebrae are in the human spine?
What is the general formula for alkanes?
What is the general formula for alkanes?
What is a characteristic of alkanes?
What is a characteristic of alkanes?
What type of reaction can alkanes undergo with oxygen?
What type of reaction can alkanes undergo with oxygen?
What is a characteristic of alkanoic acids?
What is a characteristic of alkanoic acids?
What is the general formula for alkanoic acids?
What is the general formula for alkanoic acids?
What type of reaction can alkanoic acids undergo with bases?
What type of reaction can alkanoic acids undergo with bases?
What is a type of isomerism that alkanes can exhibit?
What is a type of isomerism that alkanes can exhibit?
How are alkanes named?
How are alkanes named?
Flashcards are hidden until you start studying
Study Notes
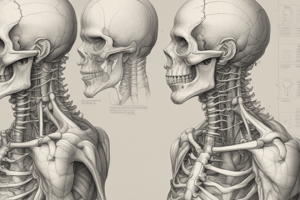
Vertebrae
Overview
- The human spine is composed of 33 vertebrae, which are divided into 5 regions: cervical, thoracic, lumbar, sacrum, and coccyx.
- Vertebrae are individual bones that are stacked on top of each other, forming the spinal column.
Characteristics
- Each vertebra has a body, pedicles, transverse processes, and a spinous process.
- The body is the main part of the vertebra, and it is the weight-bearing portion.
- Pedicles are bony projections that connect the vertebra to the posterior elements.
- Transverse processes are bony projections that serve as attachment points for muscles and ligaments.
- The spinous process is a bony projection that extends from the posterior of the vertebra.
Regions
- Cervical Vertebrae (7):
- Smaller and more delicate than other vertebrae
- Have a hole in the center for the spinal cord
- Allow for a wide range of motion
- Thoracic Vertebrae (12):
- Medium-sized and heart-shaped
- Have a smaller hole in the center for the spinal cord
- Allow for limited motion
- Lumbar Vertebrae (5):
- Larger and more robust than other vertebrae
- Have a larger hole in the center for the spinal cord
- Allow for some flexion and extension
- Sacrum (5 fused):
- Formed by the fusion of 5 vertebrae
- Triangular in shape
- Serve as a base for the spine
- Coccyx (4 fused):
- Formed by the fusion of 4 vertebrae
- Small and triangular in shape
- Serve as an attachment point for muscles and ligaments
Functions
- Support the body and maintain posture
- Protect the spinal cord and nerves
- Allow for movement and flexibility
- Serve as attachment points for muscles and ligaments
Vertebrae
Overview
- The human spine is composed of 33 vertebrae, divided into 5 regions: cervical, thoracic, lumbar, sacrum, and coccyx.
- Vertebrae are individual bones stacked on top of each other, forming the spinal column.
Characteristics
- Each vertebra has a body, pedicles, transverse processes, and a spinous process.
- The body is the main part of the vertebra, bearing weight.
- Pedicles are bony projections connecting the vertebra to the posterior elements.
- Transverse processes are bony projections serving as attachment points for muscles and ligaments.
- The spinous process is a bony projection extending from the posterior of the vertebra.
Regions
- Cervical Vertebrae (7):
- Smaller and more delicate than other vertebrae.
- Have a hole in the center for the spinal cord.
- Allow for a wide range of motion.
- Thoracic Vertebrae (12):
- Medium-sized and heart-shaped.
- Have a smaller hole in the center for the spinal cord.
- Allow for limited motion.
- Lumbar Vertebrae (5):
- Larger and more robust than other vertebrae.
- Have a larger hole in the center for the spinal cord.
- Allow for some flexion and extension.
- Sacrum (5 fused):
- Formed by the fusion of 5 vertebrae.
- Triangular in shape.
- Serve as a base for the spine.
- Coccyx (4 fused):
- Formed by the fusion of 4 vertebrae.
- Small and triangular in shape.
- Serve as an attachment point for muscles and ligaments.
Functions
- Support the body and maintain posture.
- Protect the spinal cord and nerves.
- Allow for movement and flexibility.
- Serve as attachment points for muscles and ligaments.
Alkanes
- Alkanes are saturated hydrocarbons containing only single bonds between carbon atoms.
- General formula: CnH2n+2, where n is the number of carbon atoms.
- Non-polar, making them insoluble in water.
- Have low boiling points, increasing with molecular weight.
- Generally colorless and odorless.
- Relatively unreactive, but can undergo combustion reactions with oxygen to produce carbon dioxide and water.
- Can undergo substitution reactions, where a hydrogen atom is replaced by a functional group.
- Named using the IUPAC system, identifying the longest continuous chain of carbon atoms.
- Example: a 5-carbon alkane is named pentane.
- Can exhibit structural isomerism, where molecules with the same molecular formula have different arrangements of atoms.
- Example: butane (C4H10) has two structural isomers: n-butane and isobutane.
Alkanoic Acids
- Alkanoic acids are carboxylic acids, characterized by the presence of a carboxyl (-COOH) functional group.
- General formula: CnH2n+1COOH, where n is the number of carbon atoms.
- Polar, making them soluble in water.
- Have higher boiling points than alkanes due to the presence of the polar carboxyl group.
- Weak acids, donating a proton (H+ ion) in solution.
- Can undergo neutralization reactions with bases to form salts and water.
- Can undergo esterification reactions with alcohols to form esters.
- Named using the IUPAC system, identifying the longest continuous chain of carbon atoms and adding the suffix "-oic acid".
- Example: a 5-carbon alkanoic acid is named pentanoic acid.
- Found naturally in many organisms, including plants and animals.
- Used in various applications, including food preservation, pharmaceuticals, and cosmetics.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.