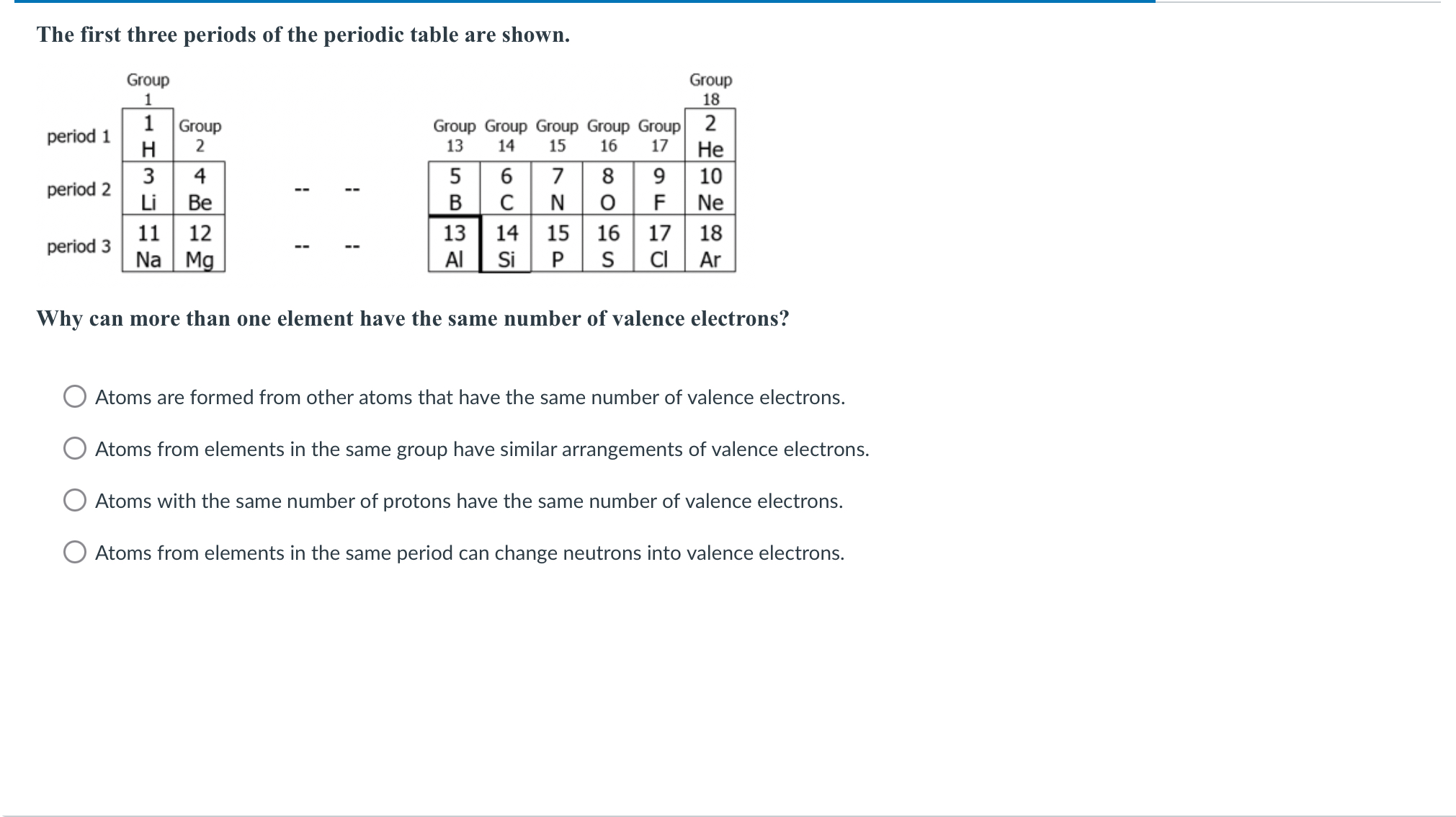
Why can more than one element have the same number of valence electrons?
Understand the Problem
The question is asking why multiple elements can have the same number of valence electrons. It presents answer choices that explore concepts related to atomic structure and periodic table groupings.
Answer
Atoms from elements in the same group have similar arrangements of valence electrons.
Atoms from elements in the same group have similar arrangements of valence electrons.
Answer for screen readers
Atoms from elements in the same group have similar arrangements of valence electrons.
More Information
Elements in the same group of the periodic table have similar chemical properties due to having the same number of valence electrons.
Sources
- ProPrep - Elements in the Same Group - proprep.com
AI-generated content may contain errors. Please verify critical information