Which one is the strongest conjugated base? PKa: A) 4.75 B) 1.29 C) 0.66 D) 2.81 E) 18
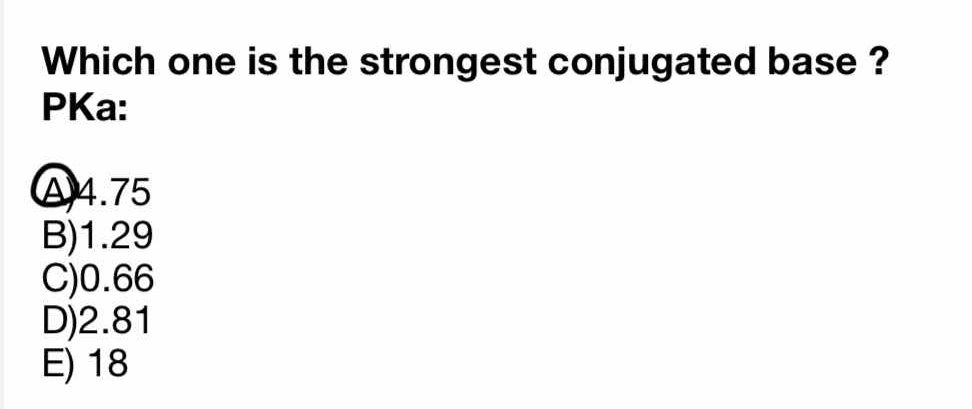
Understand the Problem
The question is asking to identify the strongest conjugated base based on the provided pKa values. The understanding is that a lower pKa value indicates a stronger acid and thus a stronger conjugate base will correspond to a higher pKa value.
Answer
The strongest conjugate base is from pKa 18.
The conjugate base from the acid with a pKa of 18 is the strongest.
Answer for screen readers
The conjugate base from the acid with a pKa of 18 is the strongest.
More Information
The acid with the highest pKa is the weakest, making its conjugate base the strongest.
Tips
A common mistake is thinking a lower pKa means a stronger base. Remember, pKa is inversely related to acid strength.
Sources
AI-generated content may contain errors. Please verify critical information