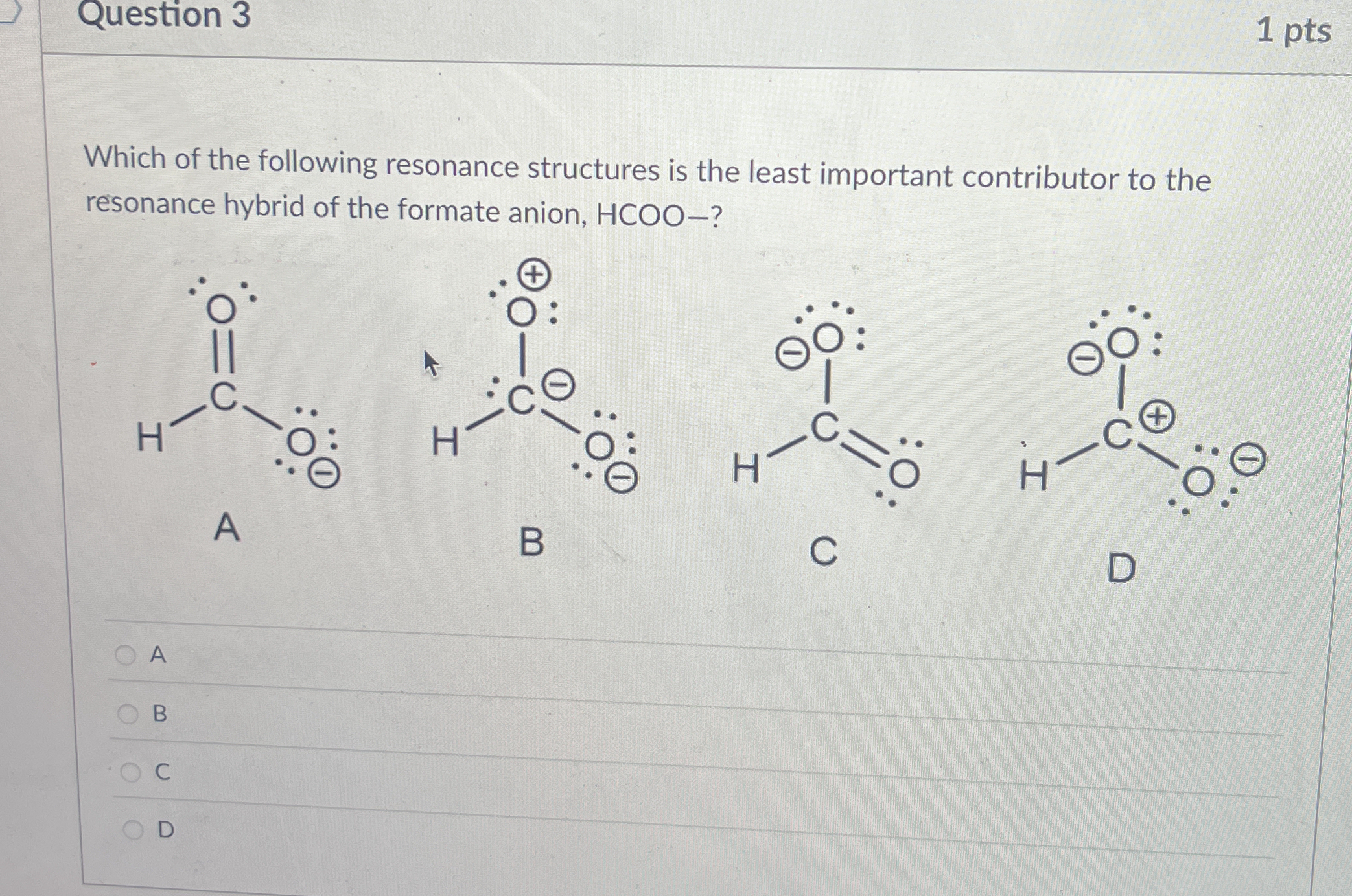
Which of the following resonance structures is the least important contributor to the resonance hybrid of the formate anion, HCOO−?
Understand the Problem
The question is asking to identify which resonance structure of the formate anion (HCOO−) is the least significant contributor to its resonance hybrid. This involves understanding resonance structures in chemistry and their relative stability and importance.
Answer
Structure B is the least important.
The least important contributor is structure B.
Answer for screen readers
The least important contributor is structure B.
More Information
Structure B has a positive charge on oxygen and a negative charge on carbon, which is less stable compared to the other resonance structures.
Tips
A common mistake is not considering the stability of the charges based on electronegativity.
Sources
AI-generated content may contain errors. Please verify critical information