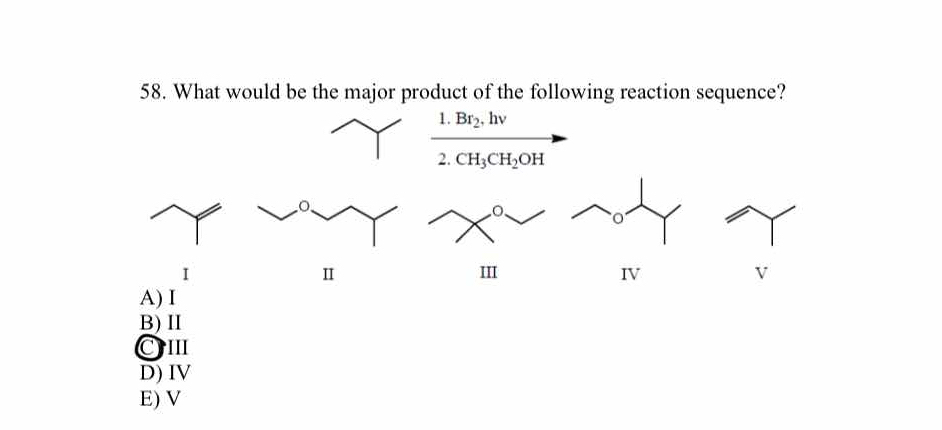
What would be the major product of the following reaction sequence? 1. Br2, hv 2. CH3CH2OH
Understand the Problem
The question is asking about the major product resulting from a specific sequence of chemical reactions involving bromination and subsequent treatment with ethanol. The user must evaluate the provided structures to determine which one corresponds to the major product of the reactions.
Answer
III
The major product of the reaction sequence is the ether shown as option III.
Answer for screen readers
The major product of the reaction sequence is the ether shown as option III.
More Information
In the first step, the Br2 with light causes homolytic cleavage which forms a radical. The most stable radical is formed on the most substituted carbon, then ethanol acts as a nucleophile replacing the bromine with an ethoxy group.
Tips
A common mistake is not recognizing the site of bromination correctly; remember that bromine adds to the most substituted carbon in radical reactions.
Sources
AI-generated content may contain errors. Please verify critical information