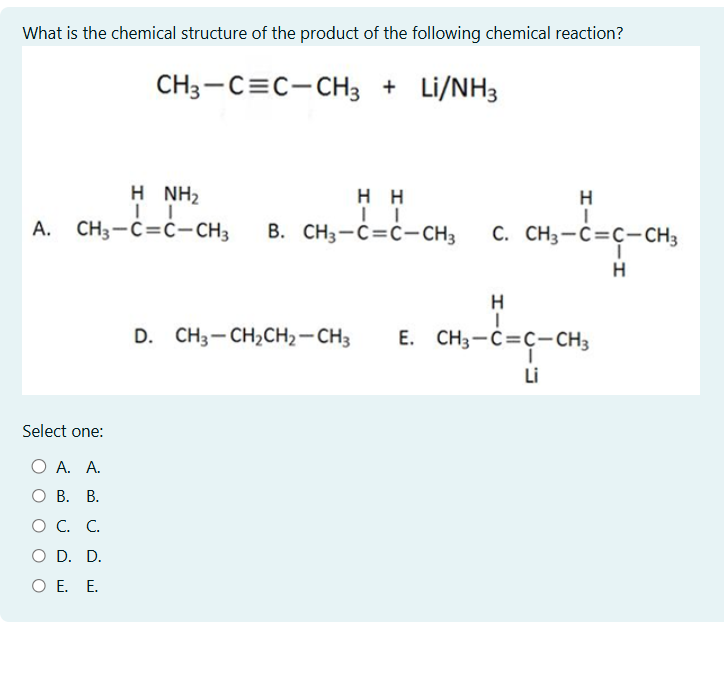
What is the chemical structure of the product of the following chemical reaction? CH3-C≡C-CH3 + Li/NH3
Understand the Problem
The question asks to identify the product of the reaction between 2-butyne (CH3-C≡C-CH3) and Lithium in liquid Ammonia (Li/NH3). This is a reduction reaction of an alkyne to a trans-alkene.
Answer
The correct answer is B. CH3-C(H)=C(H)-CH3
The correct answer is B. The reaction of an alkyne with Li/NH3 results in a trans-alkene. Therefore, the product is CH3-C(H)=C(H)-CH3
Answer for screen readers
The correct answer is B. The reaction of an alkyne with Li/NH3 results in a trans-alkene. Therefore, the product is CH3-C(H)=C(H)-CH3
More Information
The reaction of an alkyne with lithium in liquid ammonia (Li/NH3) is a classic reduction reaction that converts alkynes to trans-alkenes. This type of reaction is called a dissolving metal reduction.
Tips
A common mistake is to predict the formation of a cis-alkene or a complete reduction to an alkane. The Li/NH3 reaction is stereoselective for trans-alkenes.
Sources
- In the given reaction,CH_{3} - CHequiv C - toppr.com
- H(3)C-C-=C-CH(3)overset(Na//Liq. NH(3))rarr X In the above ... - doubtnut.com
AI-generated content may contain errors. Please verify critical information