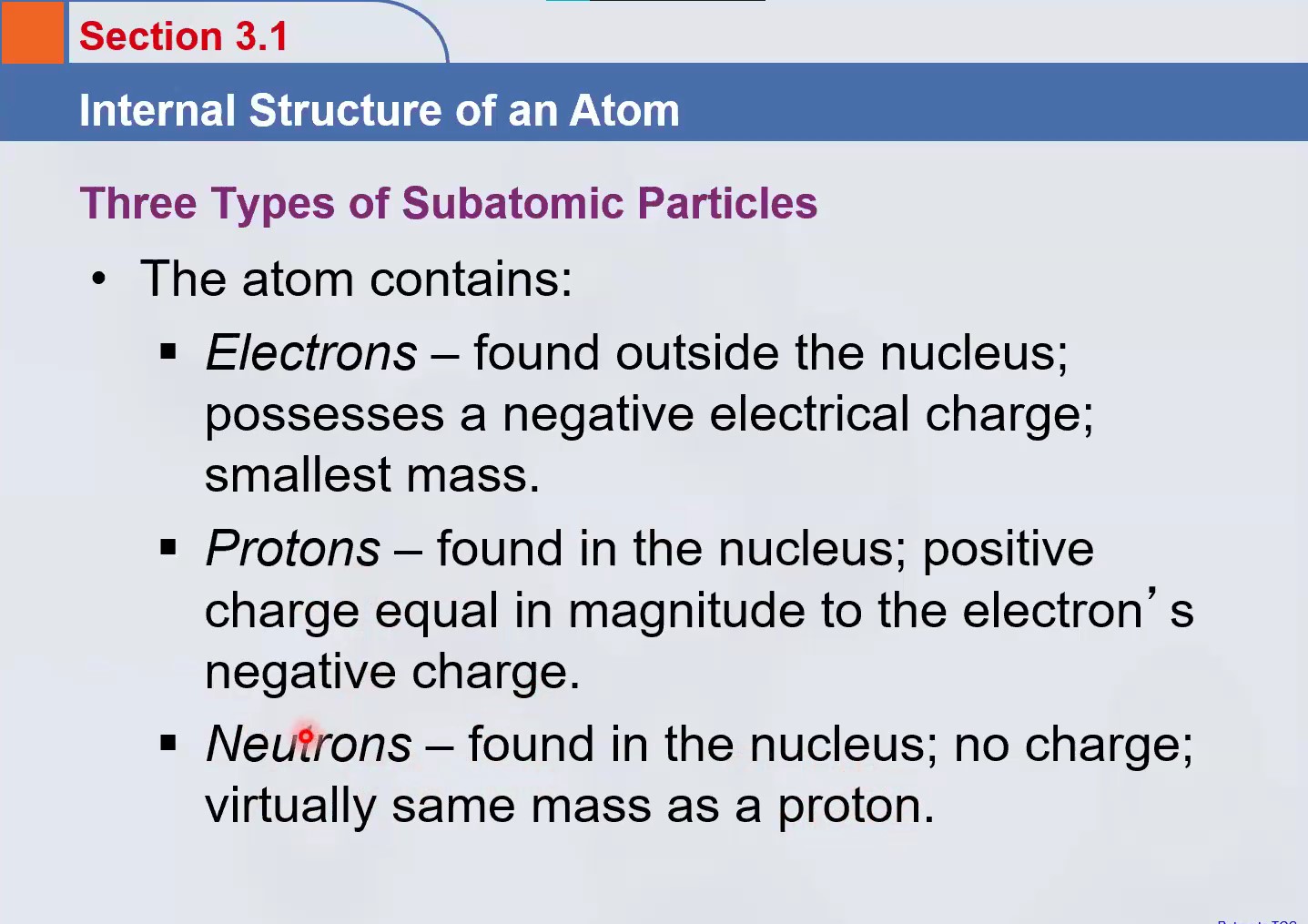
What are the three types of subatomic particles in an atom?
Understand the Problem
The image provides information about the internal structure of an atom, detailing the three types of subatomic particles: electrons, protons, and neutrons.
Answer
electrons, protons, and neutrons
The three types of subatomic particles in an atom are electrons, protons, and neutrons.
Answer for screen readers
The three types of subatomic particles in an atom are electrons, protons, and neutrons.
More Information
Electrons are found outside the nucleus with a negative charge and the smallest mass. Protons are found in the nucleus with a positive charge. Neutrons are also found in the nucleus and have no charge, with a mass nearly equal to that of a proton.
Sources
- Science 101: What is an Atom? - NRC.gov - nrc.gov
- Sub-Atomic Particles - Chemistry LibreTexts - chem.libretexts.org
- Names, charges, and locations of subatomic particles - Socratic - socratic.org
AI-generated content may contain errors. Please verify critical information