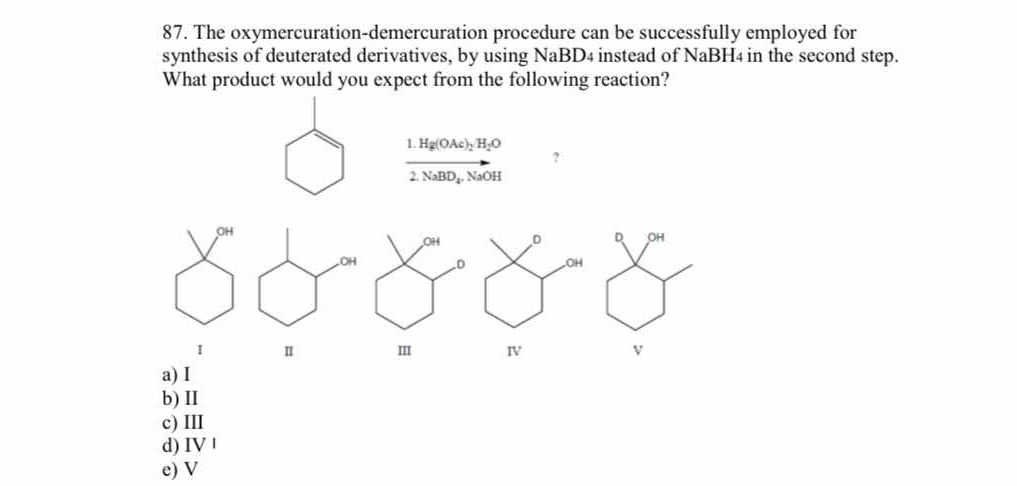
The oxymercuration-demercuration procedure can be successfully employed for synthesis of deuterated derivatives, by using NaBD4 instead of NaBH4 in the second step. What product wo... The oxymercuration-demercuration procedure can be successfully employed for synthesis of deuterated derivatives, by using NaBD4 instead of NaBH4 in the second step. What product would you expect from the following reaction?
Understand the Problem
The question is asking about the expected product from an oxymercuration-demercuration reaction when NaBD4 is used instead of NaBH4 in the second step. It presents a specific reaction setup and a choice of products (I to V), which suggests knowledge of organic chemistry reaction mechanisms.
Answer
V
The final answer is V
Answer for screen readers
The final answer is V
More Information
Using NaBD4 instead of NaBH4 introduces deuterium into the product, resulting in a deuterated alcohol.
Tips
A common mistake is forgetting that NaBD4 introduces deuterium, not hydrogen, replacing the mercury.
Sources
- Oxymercuration Demercuration of Alkenes - Master Organic Chemistry - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information