Pick the statement below that best uses the model as evidence to explain why salt water conducts electricity.
Understand the Problem
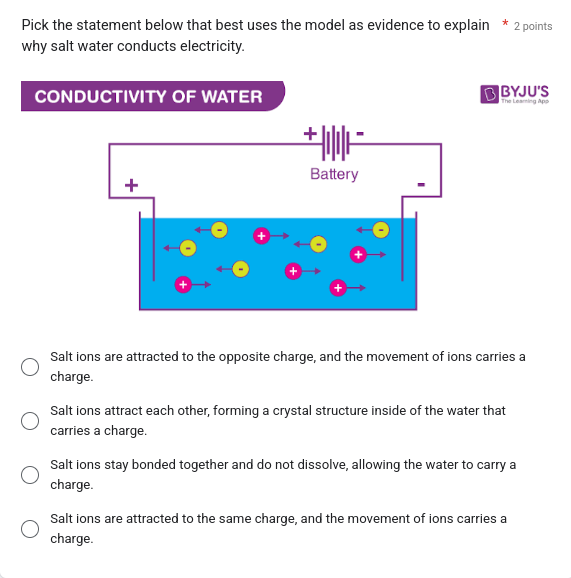
The question asks which statement best explains why salt water conducts electricity, using the provided model of ions in a solution connected to a battery as evidence. The model depicts positive and negative ions moving towards oppositely charged terminals of the battery.
Answer
Salt ions are attracted to the opposite charge, and their movement carries a charge, enabling electrical conductivity in salt water.
The best statement is: Salt ions are attracted to the opposite charge, and the movement of ions carries a charge.
Answer for screen readers
The best statement is: Salt ions are attracted to the opposite charge, and the movement of ions carries a charge.
More Information
Saltwater conducts electricity because the salt dissolves into ions (charged particles) that are free to move and carry electric charges, as shown in the image with positive ions moving towards the negative terminal and negative ions moving towards the positive terminal. This movement of ions is what creates an electric current.
Tips
A common mistake is thinking that the salt crystals themselves conduct electricity, or that ions with the same charge would attract. Remember opposite charges attract.
Sources
AI-generated content may contain errors. Please verify critical information