NH3 + __ H2SO4 → __ (NH4)2SO4
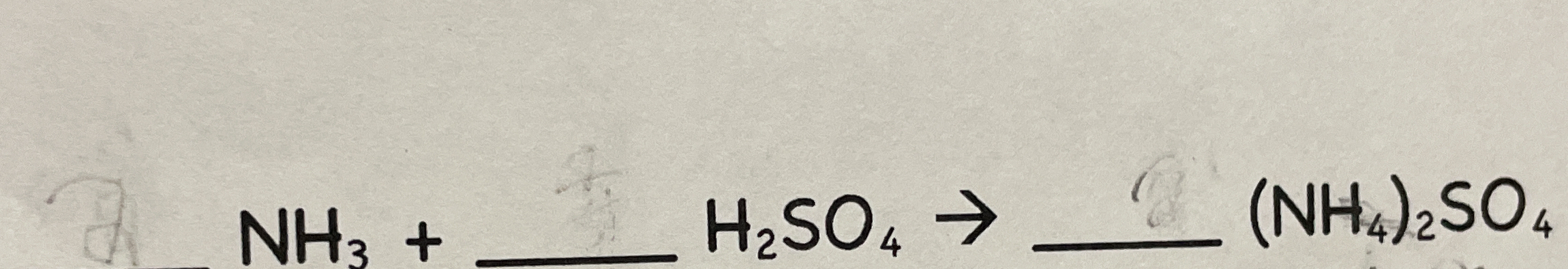
Understand the Problem
The question appears to involve balancing a chemical reaction between ammonia (NH₃) and sulfuric acid (H₂SO₄) to form ammonium sulfate ((NH₄)₂SO₄). The missing coefficients need to be identified to correctly balance the equation.
Answer
The balanced equation is: $$ 2 \text{NH}_3 + 1 \text{H}_2\text{SO}_4 \rightarrow 1 (\text{NH}_4)_2\text{SO}_4 $$
Answer for screen readers
The balanced equation is:
$$ 2 \text{NH}_3 + 1 \text{H}_2\text{SO}_4 \rightarrow 1 (\text{NH}_4)_2\text{SO}_4 $$
Steps to Solve
- Identify the compounds and elements involved
We have the reactants: ammonia ($\text{NH}_3$) and sulfuric acid ($\text{H}_2\text{SO}_4$) which form the product: ammonium sulfate ($(\text{NH}_4)_2\text{SO}_4$).
- Write down the unbalanced equation
The unbalanced equation is:
$$ \text{NH}_3 + __ \text{H}_2\text{SO}_4 \rightarrow __ (\text{NH}_4)_2\text{SO}_4 $$
- Determine the number of each atom in the products and reactants
-
On the left (reactants):
- Nitrogen: 1 (\text{NH}_3$)
- Hydrogen: 3 from $\text{NH}_3$ + 2 from $\text{H}_2\text{SO}_4$ = 5
- Sulfur: 1 ($\text{H}_2\text{SO}_4$)
- Oxygen: 4 ($\text{H}_2\text{SO}_4$)
-
On the right (products):
- Nitrogen: 2 (from $(\text{NH}_4)_2\text{SO}_4$)
- Hydrogen: 8 (from $(\text{NH}_4)_2\text{SO}_4$)
- Sulfur: 1 (from $(\text{NH}_4)_2\text{SO}_4$)
- Oxygen: 4 (from $(\text{NH}_4)_2\text{SO}_4$)
- Adjust coefficients to balance the reaction
To balance nitrogen:
- Put a coefficient of 2 in front of $\text{NH}_3$:
$$ 2 \text{NH}_3 + __ \text{H}_2\text{SO}_4 \rightarrow 1 (\text{NH}_4)_2\text{SO}_4 $$
Now recalculate:
- Nitrogen: 2 (left)
- Hydrogen: 6 (from $\text{NH}_3$) + 2 (from $\text{H}_2\text{SO}_4$) = 8 now matches product
- Sulfur: 1 now matches product
- Oxygen: 4 now matches product
- Final equation
The balanced equation is:
$$ 2 \text{NH}_3 + 1 \text{H}_2\text{SO}_4 \rightarrow 1 (\text{NH}_4)_2\text{SO}_4 $$
The balanced equation is:
$$ 2 \text{NH}_3 + 1 \text{H}_2\text{SO}_4 \rightarrow 1 (\text{NH}_4)_2\text{SO}_4 $$
More Information
This reaction signifies the formation of ammonium sulfate, a common fertilizer. Balancing chemical equations is crucial in chemistry as it adheres to the law of conservation of mass, ensuring the same number of each type of atom is present on both sides of the equation.
Tips
- Failing to count all atoms in the products and reactants.
- Not adjusting coefficients correctly to maintain balance, especially with polyatomic ions.
AI-generated content may contain errors. Please verify critical information