Is the reaction TiCl4(l) + 2H2O(l) -> TiO2(s) + 4HCl(aq) spontaneous at 25°C, given that ΔH° = -288 kJ and ΔS° = -85.4 J/K?
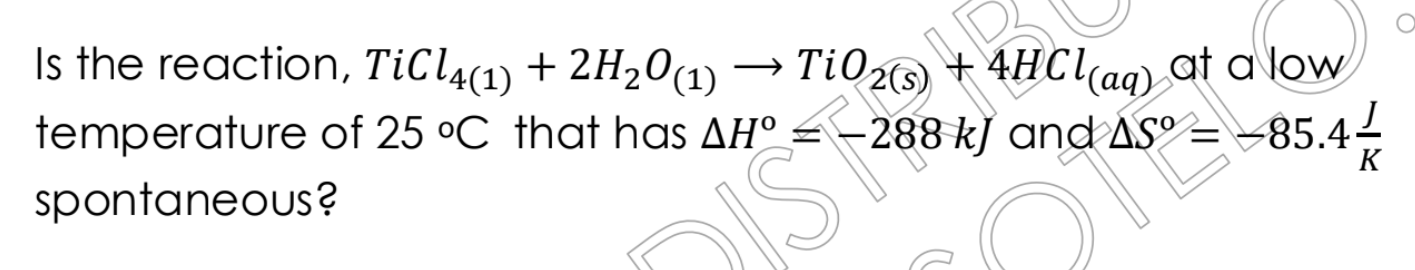
Understand the Problem
The question asks whether a given chemical reaction is spontaneous at 25°C, given the change in enthalpy (ΔH°) and the change in entropy (ΔS°). To determine spontaneity, we'll need to use the Gibbs free energy equation: ΔG = ΔH - TΔS. If ΔG is negative, the reaction is spontaneous.
Answer
Yes, the reaction is spontaneous.
Yes, the reaction is spontaneous. The Gibbs free energy change (ΔG) is negative, indicating that the reaction will occur spontaneously at 25°C.
Answer for screen readers
Yes, the reaction is spontaneous. The Gibbs free energy change (ΔG) is negative, indicating that the reaction will occur spontaneously at 25°C.
More Information
To determine spontaneity, calculate the Gibbs Free Energy (ΔG) using the equation: ΔG = ΔH - TΔS. Given: ΔH = -288 kJ = -288,000 J ΔS = -85.4 J/K T = 25°C = 298 K. Substituting these values: ΔG = -288,000 J - (298 K * -85.4 J/K) = -288,000 J + 25,449.2 J = -262,550.8 J = -262.55 kJ. Since ΔG is negative, the reaction is spontaneous at 25°C.
Tips
Pay attention to units. Make sure to convert all values to the same units before performing calculations.
Sources
- 16.4 Free Energy - Chemistry 2e | OpenStax - openstax.org
AI-generated content may contain errors. Please verify critical information