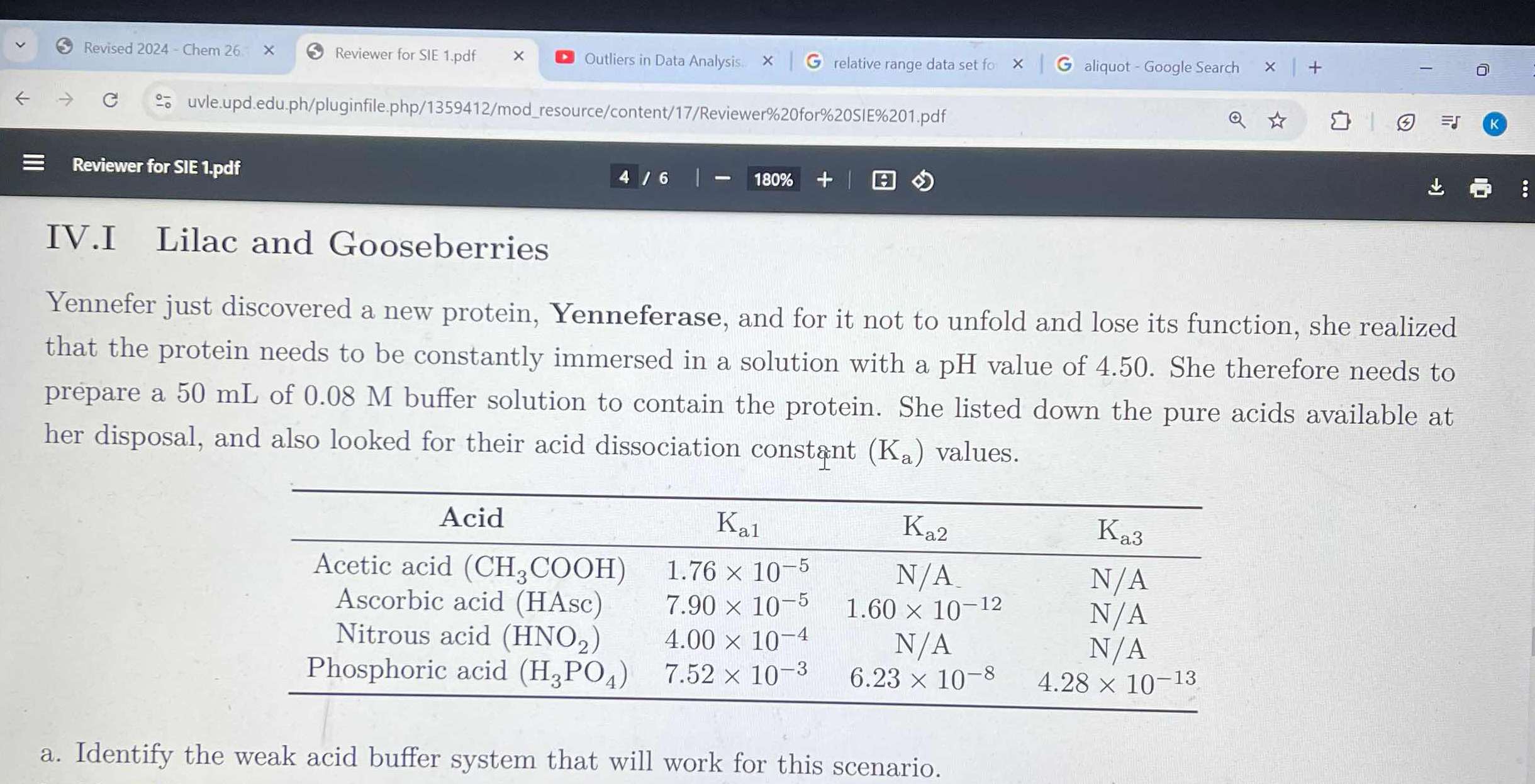
Identify the weak acid buffer system that will work for this scenario.
Understand the Problem
The problem describes a scenario where a scientist, Yennefer, needs to create a buffer solution to stabilize a newly discovered protein, Yenneferase. The protein requires a solution with a pH of 4.50 to maintain its function. Yennefer has a set of acids available and their dissociation constants (Ka) are provided. The question asks us to identify which weak acid buffer system from the provided options would be most suitable for creating a buffer with a pH of 4.50.
Answer
Nitrous acid (HNO2) with a Ka of 4.00 x 10^-4 is the most suitable weak acid for a buffer system at pH 4.50.
To determine the best weak acid buffer, calculate the pKa values (-log(Ka)) for each acid. The ideal buffer system will have a pKa close to the desired pH of 4.50. From the given options, nitrous acid (HNO2) with a Ka of 4.00 x 10^-4 (pKa = 3.40) is the most suitable choice.
Answer for screen readers
To determine the best weak acid buffer, calculate the pKa values (-log(Ka)) for each acid. The ideal buffer system will have a pKa close to the desired pH of 4.50. From the given options, nitrous acid (HNO2) with a Ka of 4.00 x 10^-4 (pKa = 3.40) is the most suitable choice.
More Information
A buffer solution works best when the pKa of the weak acid is close to the desired pH of the buffer, typically within ± 1 pH unit.
Tips
A common mistake is selecting an acid with a Ka value closest to the pH instead of considering the pKa value. Always convert Ka to pKa first.
Sources
- 7.1: Acid-Base Buffers - Chemistry LibreTexts - chem.libretexts.org
- Which combination of solutions is the best choice for making a buffer ... - Quora - quora.com
AI-generated content may contain errors. Please verify critical information