How strong are acid or base solutions?
Understand the Problem
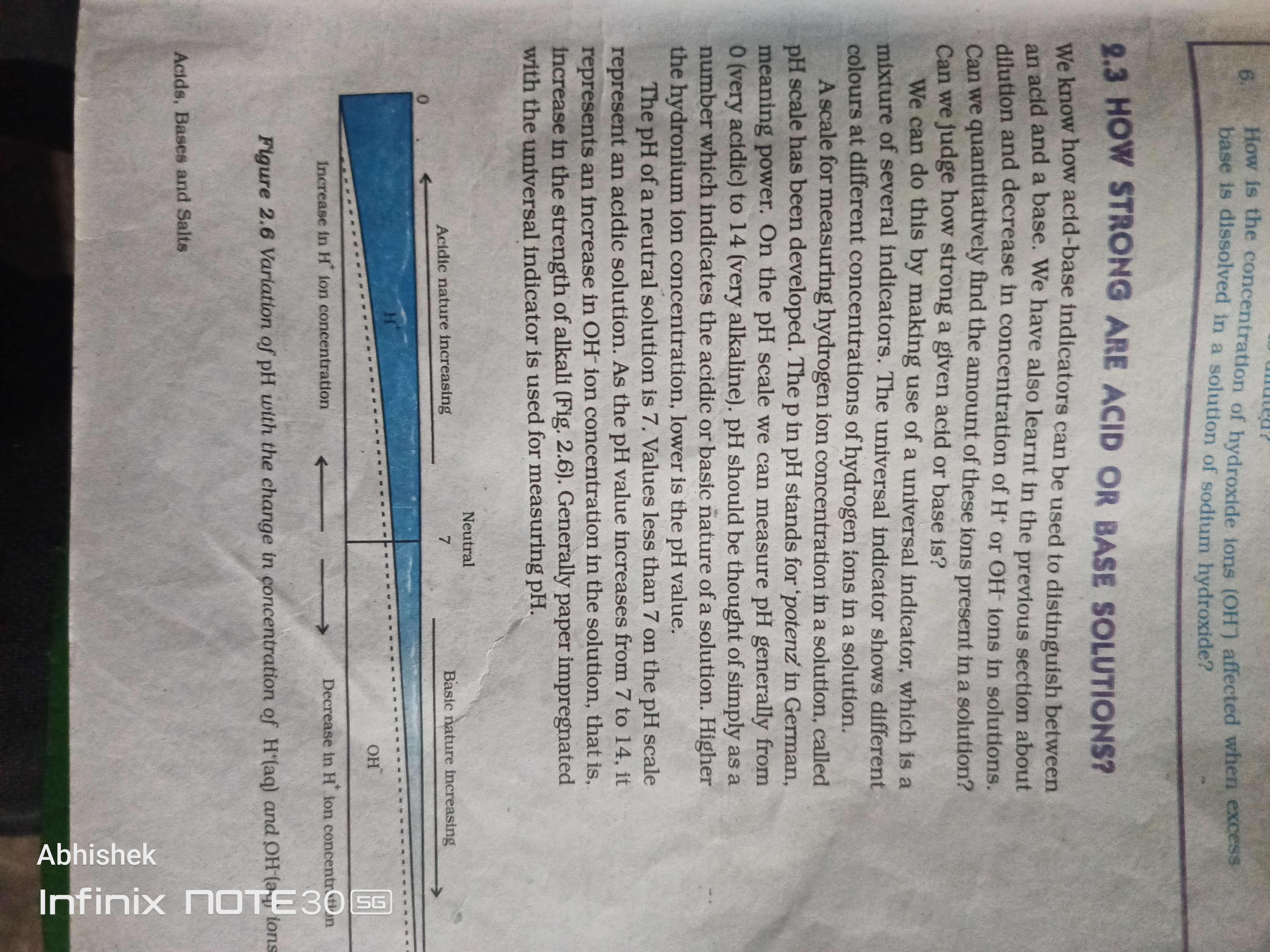
The text discusses how strong acid or base solutions can be determined using pH indicators and includes information about the pH scale and the concentration of hydrogen ions in solutions.
Answer
Strong acids: pH 0-1, strong bases: pH 12-14.
The strength of acid or base solutions is determined by the pH scale. Strong acids have a pH of 0 to 1, while strong bases have a pH of 12 to 14.
Answer for screen readers
The strength of acid or base solutions is determined by the pH scale. Strong acids have a pH of 0 to 1, while strong bases have a pH of 12 to 14.
More Information
pH is a logarithmic scale used to specify the acidity or basicity of an aqueous solution. pH values below 7 represent acidic solutions, while values above 7 indicate basic solutions.
Tips
A common mistake is confusing strong and concentrated solutions. Strength refers to the degree of ionization, not concentration.
Sources
- Strong Acids and Bases in Chemistry - byjus.com
- Acid and Base Strength - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information