Give the systematic name for the following coordination compound or complex ion:
Understand the Problem
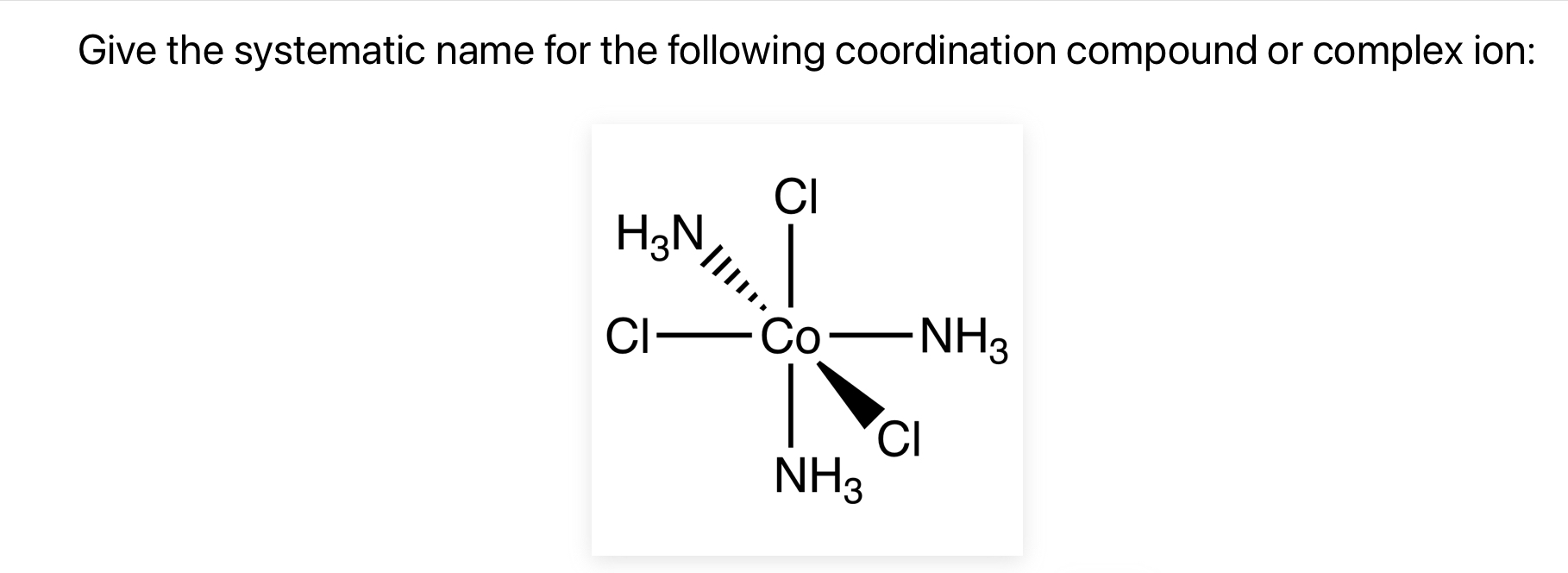
The question is asking for the systematic (IUPAC) name of the coordination compound or complex ion depicted in the image. Specifically, the complex consists of a central cobalt (Co) ion coordinated with three chloride (Cl) ligands and three ammonia (NH3) ligands. To name the complex, one must follow IUPAC nomenclature rules for coordination compounds, considering the ligands, their alphabetical order, quantity, and the oxidation state of the central metal ion.
Answer
Triamminetrichlorocobalt(III)
The systematic name for the coordination compound [Co(NH3)3Cl3] is triamminetrichlorocobalt(III).
Answer for screen readers
The systematic name for the coordination compound [Co(NH3)3Cl3] is triamminetrichlorocobalt(III).
More Information
The complex has a neutral charge, so it's named as a coordination compound rather than an ion. We name ligands first in alphabetical order. ammine before chloro. Since there are three of each, we use the prefixes 'tri'. Finally, we indicate the oxidation state of cobalt as (III).
Tips
Common mistakes include not alphabetizing ligands correctly or misidentifying the oxidation state of the metal center.
Sources
- Nomenclature of Coordination Complexes - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information