Follow the format of activities with margin = Long Bond Paper. Title: Balancing & Equation for group work. 1. Al + FeO → Al2O3 + Fe. 2. P4 + O2 → P2O5. 3. K2O + H2O → KOH. 4. Na2O2... Follow the format of activities with margin = Long Bond Paper. Title: Balancing & Equation for group work. 1. Al + FeO → Al2O3 + Fe. 2. P4 + O2 → P2O5. 3. K2O + H2O → KOH. 4. Na2O2 + H2O → NaOH + O. 5. C + H2O → CO + H. 6. H3AsO4 + H2O → As2O5 + HCl.
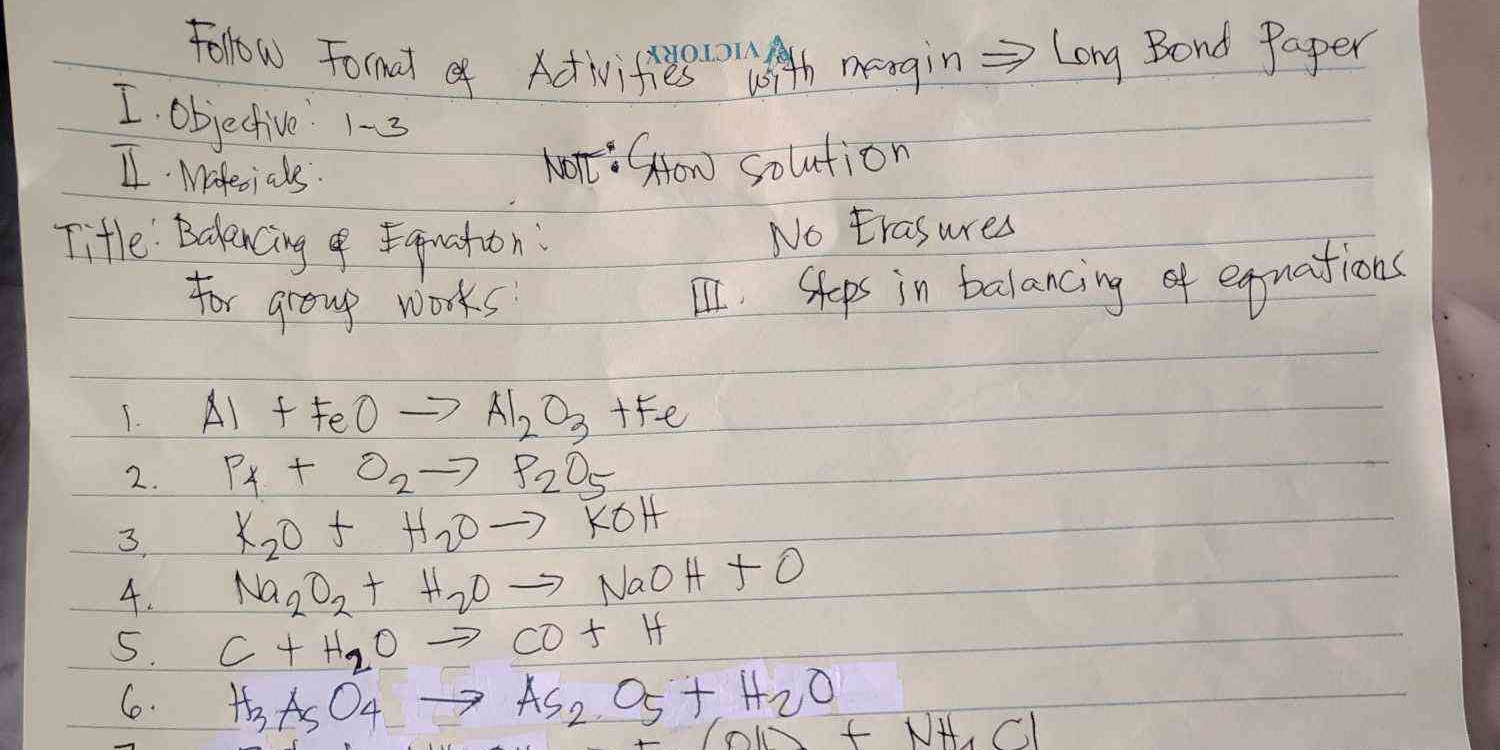
Understand the Problem
The document outlines an activity focused on balancing chemical equations. It includes instructions for group work, a title, materials needed, and a series of chemical reactions to balance. The format suggests a structured approach to conducting an experiment or a lesson.
Answer
Balanced equations: 1. 2Al + 3FeO → Al2O3 + 3Fe 2. P4 + 5O2 → 2P2O5 3. K2O + H2O → 2KOH 4. 2Na2O2 + 2H2O → 4NaOH + O2 5. C + H2O → CO + H2 6. H3AsO4 + 3H2O → 1.5As2O5 + 6HCl
The balanced equations are:
- 2Al + 3FeO → Al2O3 + 3Fe
- P4 + 5O2 → 2P2O5
- K2O + H2O → 2KOH
- 2Na2O2 + 2H2O → 4NaOH + O2
- C + H2O → CO + H2
- H3AsO4 + 3H2O → 1.5As2O5 + 6HCl
Answer for screen readers
The balanced equations are:
- 2Al + 3FeO → Al2O3 + 3Fe
- P4 + 5O2 → 2P2O5
- K2O + H2O → 2KOH
- 2Na2O2 + 2H2O → 4NaOH + O2
- C + H2O → CO + H2
- H3AsO4 + 3H2O → 1.5As2O5 + 6HCl
More Information
Balancing chemical equations ensures the same number of atoms of each element are present on both sides of the reaction. This follows the law of conservation of mass.
Tips
Ensure each element has the same number of atoms on both sides. Check diatomic molecules like O2, and simplify ratios if possible.
AI-generated content may contain errors. Please verify critical information