Find mass of 117.2 ml of N2 gas at STP.
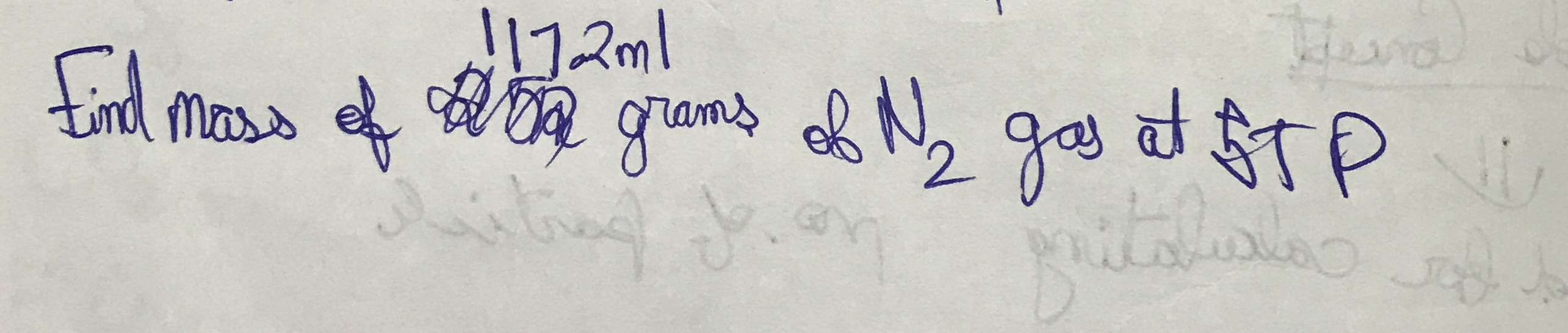
Understand the Problem
The question is asking to calculate the mass of a specific volume of nitrogen gas (N2) at standard temperature and pressure (STP). The calculation requires understanding the relationship between volume, mass, and the conditions of the gas.
Answer
The mass of 117.2 mL of nitrogen gas at STP is approximately $0.146 \, \text{g}$.
Answer for screen readers
The mass of 117.2 mL of N₂ gas at STP is approximately $0.146 , \text{g}$.
Steps to Solve
- Convert volume from milliliters to liters
To use the ideal gas law, first convert the volume of nitrogen gas from milliliters (mL) to liters (L).
$$ 117.2 , \text{mL} = 0.1172 , \text{L} $$
- Use the ideal gas law to find moles
At Standard Temperature and Pressure (STP), 1 mole of gas occupies 22.4 L.
Calculate the number of moles of nitrogen gas using the formula:
$$ \text{moles} = \frac{\text{volume}}{\text{molar volume at STP}} $$
So,
$$ \text{moles of } N_2 = \frac{0.1172 , \text{L}}{22.4 , \text{L/mol}} $$
- Calculate the mass of nitrogen gas
The molar mass of nitrogen gas (N₂) is approximately 28.02 g/mol.
Using the number of moles calculated in the previous step, find the mass:
$$ \text{mass} = \text{moles} \times \text{molar mass} $$
- Substitute the values and calculate the mass
Substituting the calculated moles into the equation:
$$ \text{mass of } N_2 = \left( \frac{0.1172 , \text{L}}{22.4 , \text{L/mol}} \right) \times 28.02 , \text{g/mol} $$
- Final calculation
Now perform the final computation:
$$ \text{mass of } N_2 \approx (0.00523) \times 28.02 \approx 0.146 , \text{g} $$
The mass of 117.2 mL of N₂ gas at STP is approximately $0.146 , \text{g}$.
More Information
At STP, nitrogen gas behaves ideally, allowing the calculation of mass directly from volume. This is useful in various scientific applications, especially in chemistry and physics.
Tips
- Confusing milliliters with liters: Always ensure you convert volumes to the correct units.
- Incorrectly using molar mass: Confirm you are using the molar mass of nitrogen (28.02 g/mol) and not just nitrogen's atomic mass.
AI-generated content may contain errors. Please verify critical information