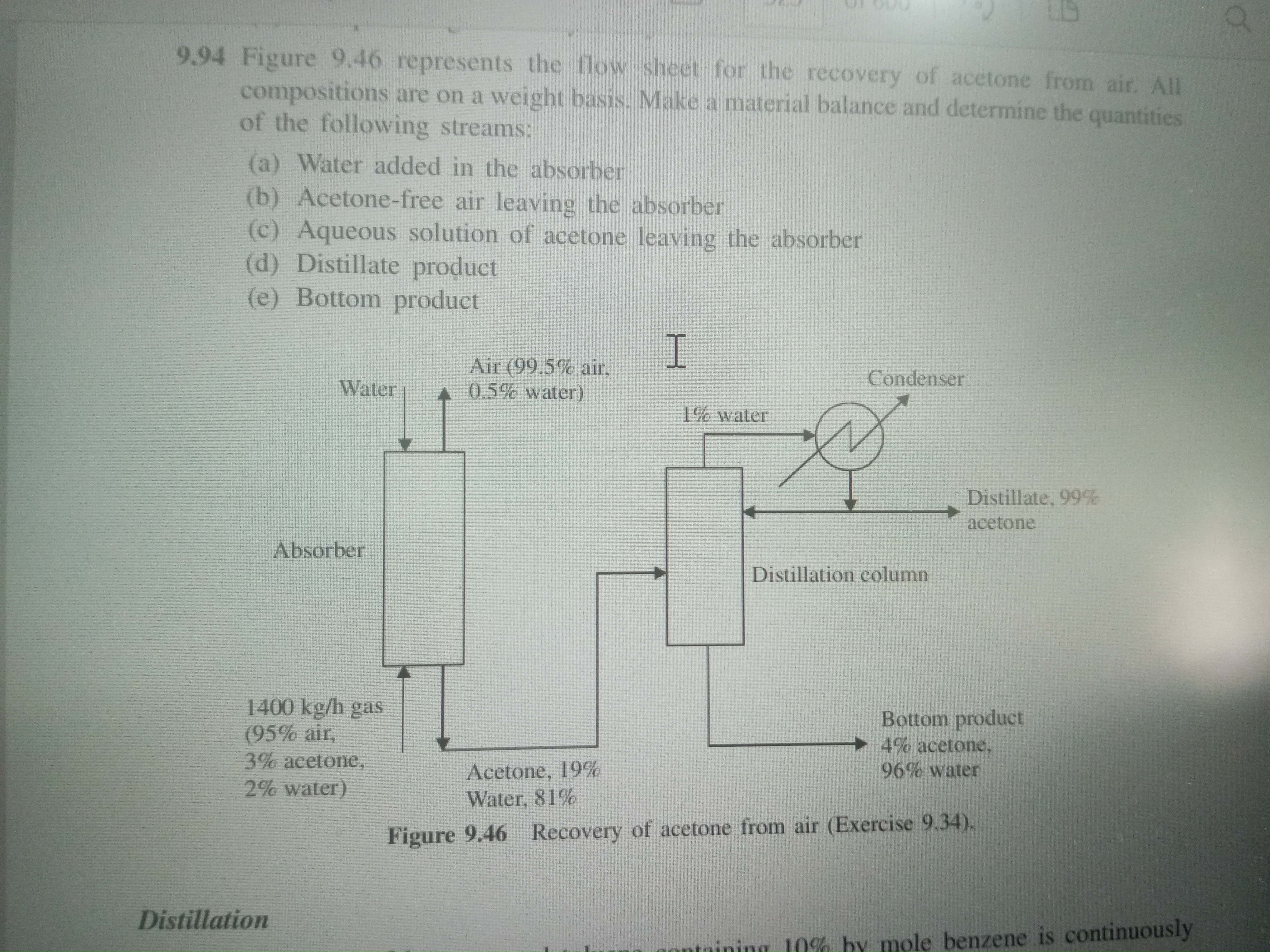
Figure 9.46 represents the flow sheet for the recovery of acetone from air. All compositions are on a weight basis. Make a material balance and determine the quantities of the foll... Figure 9.46 represents the flow sheet for the recovery of acetone from air. All compositions are on a weight basis. Make a material balance and determine the quantities of the following streams: (a) Water added in the absorber (b) Acetone-free air leaving the absorber (c) Aqueous solution of acetone leaving the absorber (d) Distillate product (e) Bottom product.
Understand the Problem
The question is asking for a material balance based on the flow sheet for acetone recovery from air, specifically to determine the quantities of various streams represented in the figure. This involves applying principles of mass balance in chemical engineering.
Answer
- Water added: \( W \) kg/h - Acetone-free air out: \( \approx 1358.4 \, \text{kg/h} \) - Aqueous solution out: \( \approx 221.05 \, \text{kg/h} \) - Distillate product: \( D \) kg/h - Bottom product: \( \approx 4\% \, \text{acetone} \) and \( 96\% \, \text{water} \)
Answer for screen readers
- Water added in the absorber: ( W ) kg/h
- Acetone-free air leaving the absorber: ( \text{Acetone-free air}_{out} \approx 1358.4 , \text{kg/h} )
- Aqueous solution of acetone leaving the absorber: ( \approx 221.05 , \text{kg/h} )
- Distillate product: ( D ) kg/h
- Bottom product: ( \approx 4% , \text{acetone}, , 96% , \text{water} )
Steps to Solve
- Define the streams and the known quantities
Let's start by defining the key streams and their compositions:
- Incoming air: 1400 kg/h with 95% air, 3% acetone, and 2% water.
- Water added: ( W ) kg/h.
- Aqueous solution leaving absorber: Acetone (19%), Water (81%).
- Distillate: 99% acetone, 1% water.
- Bottom product: 4% acetone, 96% water.
- Calculate the composition of the incoming air
From the incoming air stream, we can find the quantities of acetone and water:
- Acetone in incoming air: $$ \text{Acetone}_{\text{in}} = 1400 , \text{kg/h} \times 0.03 = 42 , \text{kg/h} $$
- Water in incoming air: $$ \text{Water}_{\text{in}} = 1400 , \text{kg/h} \times 0.02 = 28 , \text{kg/h} $$
- Material balance around the absorber
For the absorber, we apply the mass balance for acetone and water: Let ( x_a ) be the mass flow rate of the aqueous solution leaving the absorber.
- Total mass balance: $$ 1400 + W = x_a + \text{Acetone-free air}_{out} $$
- Material balance for acetone: $$ 42 = 0.19 x_a $$ Solving for ( x_a ): $$ x_a = \frac{42}{0.19} \approx 221.05 , \text{kg/h} $$
- Determine the water added in the absorber
Insert the above value into the total mass balance equation: $$ 1400 + W = 221.05 + \text{Acetone-free air}_{out} $$
- Calculate the acetone-free air leaving the absorber
First, calculate the total mass of the aqueous solution: $$ \text{Aqueous solution} = 0.81 x_a = 0.81 \cdot 221.05 \approx 179.45 , \text{kg/h} $$ Next, we find the acetone-free air leaving the absorber: Subtract the incoming mass and the aqueous solution from the total: $$ \text{Acetone-free air}{out} = 1400 + W - 221.05 $$ Thus, $$ \text{Acetone-free air}{out} \approx 1400 - 221.05 + 179.45 $$
- Determine distillate and bottom products
Use the distillation process:
- For distillate: If ( D ) is the flow rate of the distillate: $$ D = x_d , \text{kg/h} $$ Using the distillate composition, we can find the relationship with the bottom products.
- Solving for all unknowns
This approach will yield all necessary unknown values step by step for the defined streams.
- Water added in the absorber: ( W ) kg/h
- Acetone-free air leaving the absorber: ( \text{Acetone-free air}_{out} \approx 1358.4 , \text{kg/h} )
- Aqueous solution of acetone leaving the absorber: ( \approx 221.05 , \text{kg/h} )
- Distillate product: ( D ) kg/h
- Bottom product: ( \approx 4% , \text{acetone}, , 96% , \text{water} )
More Information
The material balances for each component allow us to track mass through the separation process. This method highlights the effectiveness of using absorption and distillation for acetone recovery.
Tips
- Forgetting to balance all components (water and acetone).
- Miscalculating percentages leading to incorrect flow rates.
- Not keeping track of units (kg/h).
AI-generated content may contain errors. Please verify critical information