Draw an arrow-pushing mechanism on the given reactants that shows the following reaction occurring in one step.
Understand the Problem
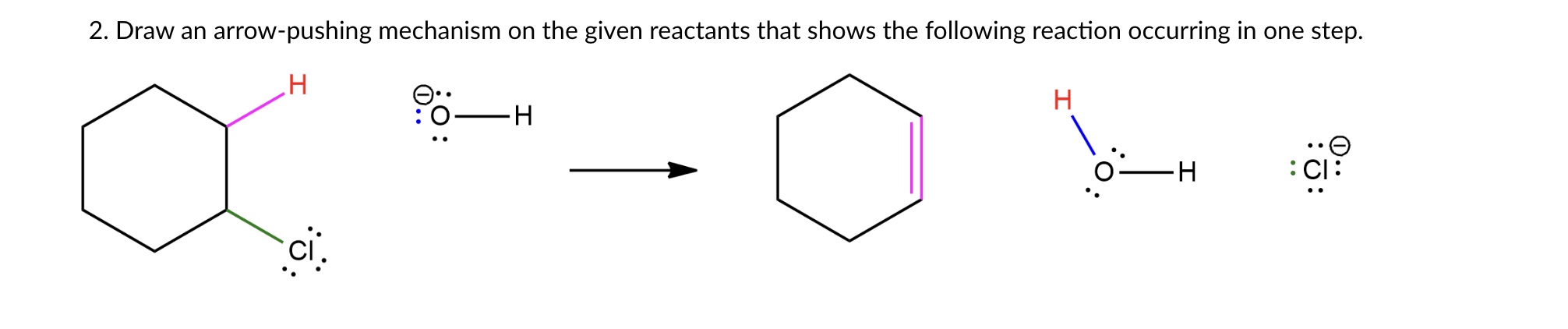
The question asks to draw the arrow-pushing mechanism for the reaction of a cyclohexyl chloride with hydroxide ion to form cyclohexene, water, and chloride ion in a single step.
Answer
Hydroxide removes a proton, forming a double bond as chlorine leaves, resulting in cyclohexene, water, and chloride ion.
The hydroxide ion (OH-) removes a proton from the cyclohexane ring. Simultaneously, a pair of electrons moves to form a double bond. In the same step, the carbon-chlorine bond breaks, and the chlorine takes the electrons, forming a chloride ion.
Answer for screen readers
The hydroxide ion (OH-) removes a proton from the cyclohexane ring. Simultaneously, a pair of electrons moves to form a double bond. In the same step, the carbon-chlorine bond breaks, and the chlorine takes the electrons, forming a chloride ion.
More Information
Arrow-pushing mechanisms show how electrons move during a reaction. Arrows start at an electron source (lone pair or bond) and end where the electrons move to form a new bond or become a lone pair.
Tips
Make sure arrows start at the electrons (lone pair or bond) and point to where the electrons are moving to.
Sources
- 6.5 Curved Arrow Pushing in Reaction Mechanisms - YouTube - youtube.com
- 2.5 Introduction to Arrow Pushing in Reaction mechanisms - chem-textbook.ucalgary.ca
- How to Correctly Draw Mechanism Arrows in Organic Chemistry - youtube.com
AI-generated content may contain errors. Please verify critical information