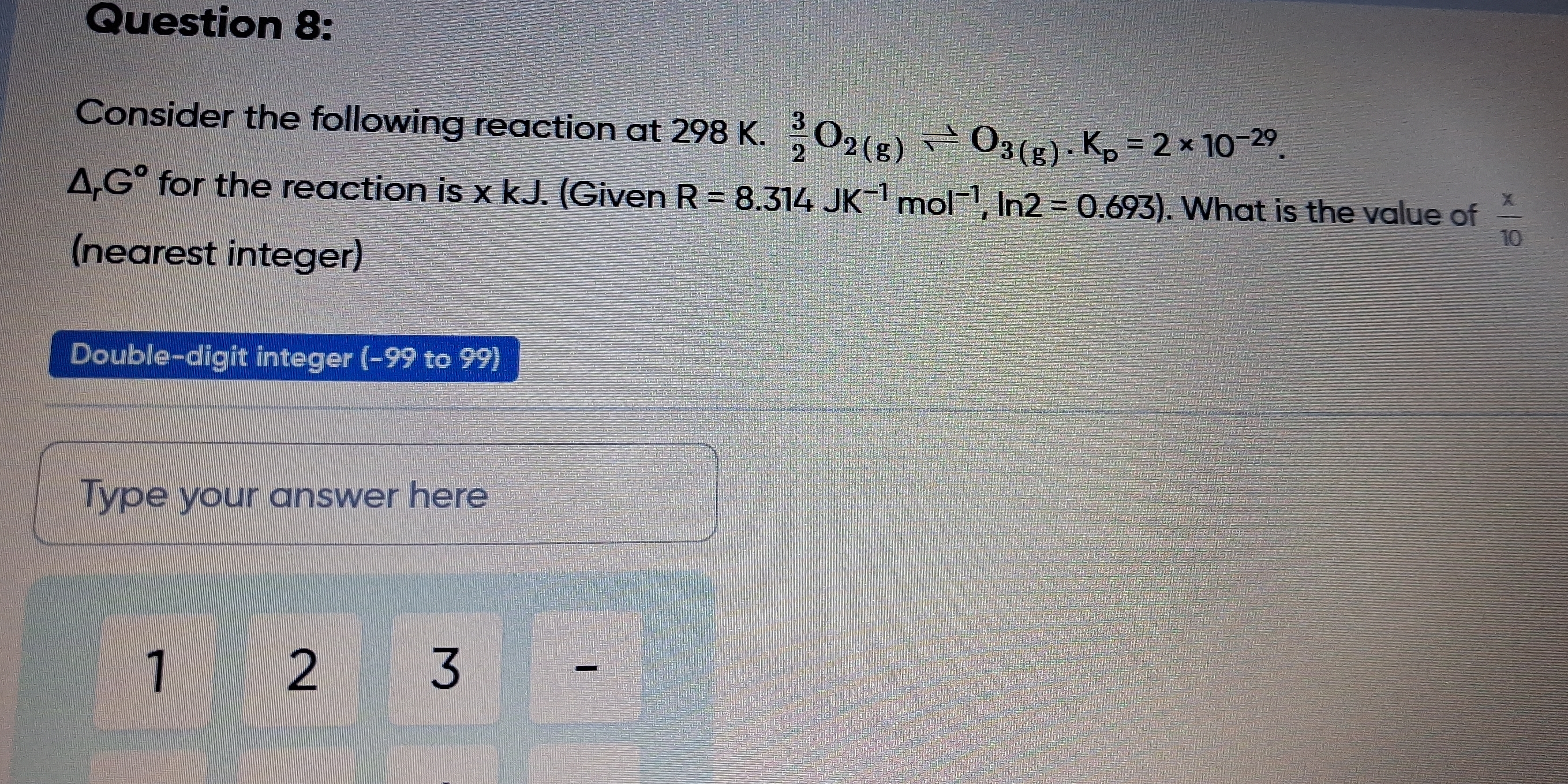
Consider the following reaction at 298 K. ΔG° for the reaction is x kJ. (Given R = 8.314 JK⁻¹ mol⁻¹, ln2 = 0.693). What is the value of x/10 (nearest integer)?
Understand the Problem
The question is asking for the standard Gibbs free energy change (ΔG°) for a given reaction at a specified temperature and equilibrium constant. The user must calculate this value and express it as a double-digit integer divided by 10, rounding to the nearest integer.
Answer
16
Answer for screen readers
The value of ( \frac{x}{10} ) is 16.
Steps to Solve
-
Identify the given values The equilibrium constant ( K_p = 2 \times 10^{-29} ) and the temperature ( T = 298 , K ). The values for the gas constant are ( R = 8.314 , J , K^{-1} , mol^{-1} ) and ( \ln(2) = 0.693 ).
-
Convert ( K_p ) to the correct units Since ( K_p ) is a pressure equilibrium constant, we will need to use it directly.
-
Calculate ( \Delta G^\circ ) using the equation The equation to calculate standard Gibbs free energy change is:
$$ \Delta G^\circ = -RT \ln K $$
Substituting in the known values:
$$ \Delta G^\circ = - (8.314 , J , K^{-1} , mol^{-1}) \times 298 , K \times \ln(2 \times 10^{-29}) $$
- Calculate ( \ln K ) Use the properties of logarithms to find ( \ln(2 \times 10^{-29}) ):
$$ \ln(2 \times 10^{-29}) = \ln(2) + \ln(10^{-29}) = 0.693 - 29 \ln(10) $$
Using ( \ln(10) \approx 2.303 ):
$$ \ln(2 \times 10^{-29}) \approx 0.693 - 29 \times 2.303 $$
Now calculate ( 29 \times 2.303 ):
$$ 29 \times 2.303 \approx 66.787 $$
So now we have:
$$ \ln(2 \times 10^{-29}) \approx 0.693 - 66.787 \approx -66.094 $$
- Substitute ( \ln K ) back into the Gibbs free energy equation Now substitute ( \ln K ) back to find ( \Delta G^\circ ):
$$ \Delta G^\circ \approx - (8.314) \times 298 \times (-66.094) $$
Calculating this:
$$ \Delta G^\circ \approx 8.314 \times 298 \times 66.094 $$
Calculating step-by-step:
- First calculate ( 8.314 \times 298 \approx 2477.572 )
Then multiply by ( 66.094 ):
$$ \Delta G^\circ \approx 2477.572 \times 66.094 \approx 163,323.9 , J = 163.32 , kJ $$
- Calculate ( \frac{x}{10} ) and round Now divide by 10:
$$ \frac{163.32}{10} \approx 16.332 $$
Rounding to the nearest integer gives ( 16 ).
The value of ( \frac{x}{10} ) is 16.
More Information
The Gibbs free energy change is a useful value in thermodynamics that helps determine the spontaneity of a reaction. A negative ( \Delta G^\circ ) indicates that the reaction is spontaneous under standard conditions.
Tips
- Forgetting to convert ( K_p ) to the logarithmic form correctly.
- Miscalculating the multiplication of constants and the logarithmic value.
- Not rounding properly at the end.
AI-generated content may contain errors. Please verify critical information